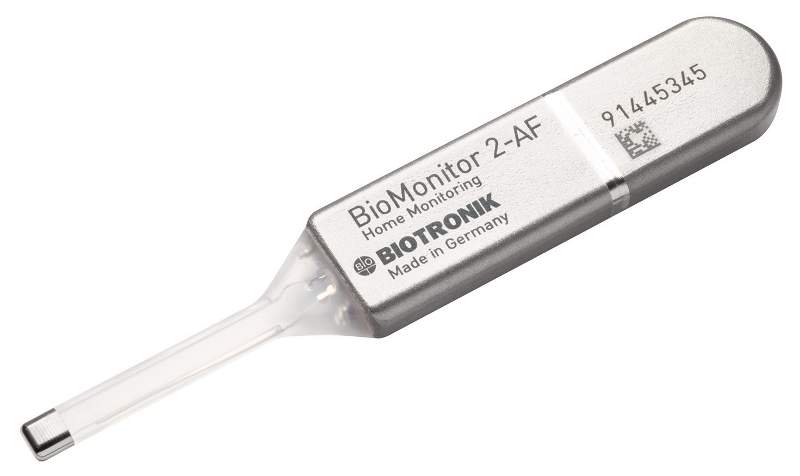

BIOTRONIK has started enrolling patients for its BioInsight clinical study of the BioMonitor 2 insertion procedure in an office setting.
BioMonitor 2 is an insertable cardiac remote monitor based on ProMRI technology, it is placed underneath a patient's skin to track evidence of suspected cardiac arrhythmia or unexplained syncope.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The insertable cardiac monitor has been designed to facilitate accurate detection of arrhythmias by delivering good signal quality.
BioMonitor 2 enables reliable and continuous arrhythmia monitoring with automatic, daily transmissions and highly efficient alert management.
The device can also be used for the monitoring of atrial fibrillation in patients who have undergone ablation procedures.
The BioInsight study is being conducted as a multi-centre, prospective, non-randomised post-market study intended to assess the safety and feasibility of performing the minimally invasive BioMonitor 2 insertion procedure in an office setting.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataDuring the study, the subjects will receive BioMonitor 2 via in-office insertion and will be examined for 90 days to monitor for any potential adverse events, including infection and bleeding.
BIOTRONIK president Marlou Janssen said: "BioMonitor 2 has been rapidly adopted by physicians, becoming a trusted, reliable solution to monitor for cardiac arrhythmias.
"There is a significant need for BioMonitor 2, and we want to ensure our physicians have the utmost confidence in their ability to deliver efficient patient care.
“The BioInsight study will assure physicians and patients that performing the insertion procedure in an office setting safely and effectively improves access to this critical diagnostic tool."
The study is slated to be completed in the third quarter of next year.
Image: The BioMonitor 2. Photo: courtesy of BIOTRONIK.





