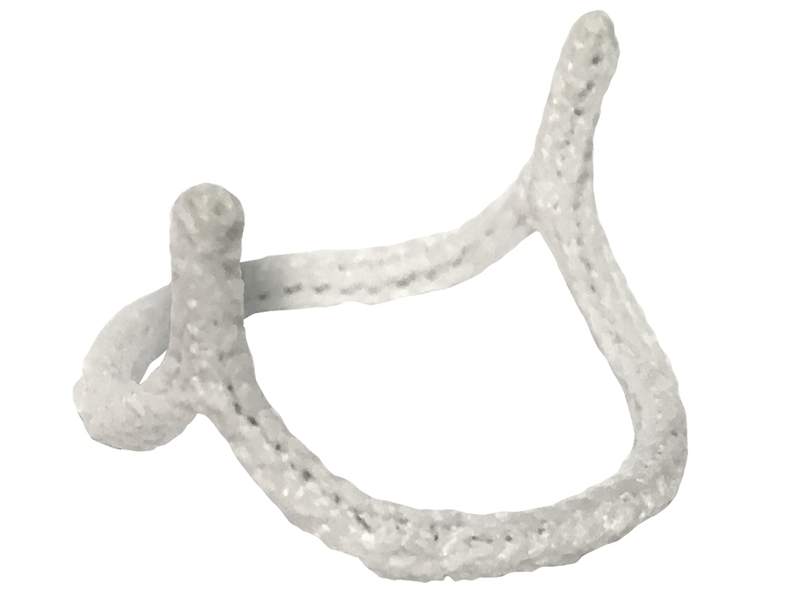

The US Food and Drug Administration (FDA) has granted market clearance to cardiovascular devices firm BioStable Science and Engineering’s HAART 200 Aortic Annuloplasty Device.
Developed for bicuspid aortic valve (BAV) repair, the HAART 200 device targets patients with aortic valve insufficiency caused due to BAV.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
BAV is a congenital malformation that occurs when aortic valve forms only two functional valve leaflets and is said to result in cardiovascular complications such as aneurysms and aorta dissections.
The majority of BAV patients are reported to require aortic valve replacement, which increases the risk for reoperation or related complications.
Aortic valve repair is intended to act as a surgical alternative to such patients and is expected to improve outcomes when compared to valve replacement.
The HAART 200 Aortic Annuloplasty Device is developed to minimise annular diameter based on leaflet size, conform the annulus to a circular symmetric shape for improved valve function, and for providing long-term stabilisation to the annular geometry.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBioStable Science and Engineering president and CEO John Wheeler said: “Valve repair is an especially attractive alternative for the young population of patients requiring surgical intervention for BAV disease.
“HAART 200 is uniquely designed to address the specific technical challenges of BAV repair.”
The firm also previously obtained FDA clearance for its HAART 300 Aortic Annuloplasty Device designed to minimise annular dilatation, restore three-dimensional annular geometry and act as a framework for leaflet repair procedure guidance.
Image: HAART 200 Aortic Annuloplasty Device. Photo: courtesy of BioStable Science and Engineering.





