
Ireland-based Medtronic's Resolute Onyx drug-eluting stent (DES) has received approval from the US Food and Drug Administration (FDA).
Based on the performance and deliverability of the Resolute Integrity DES, the new device is designed to improve the safety and efficacy of DES technology.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The new tool is available in sizes 4.5mm and 5mm to allow physicians to treat patients with large coronary anatomies.
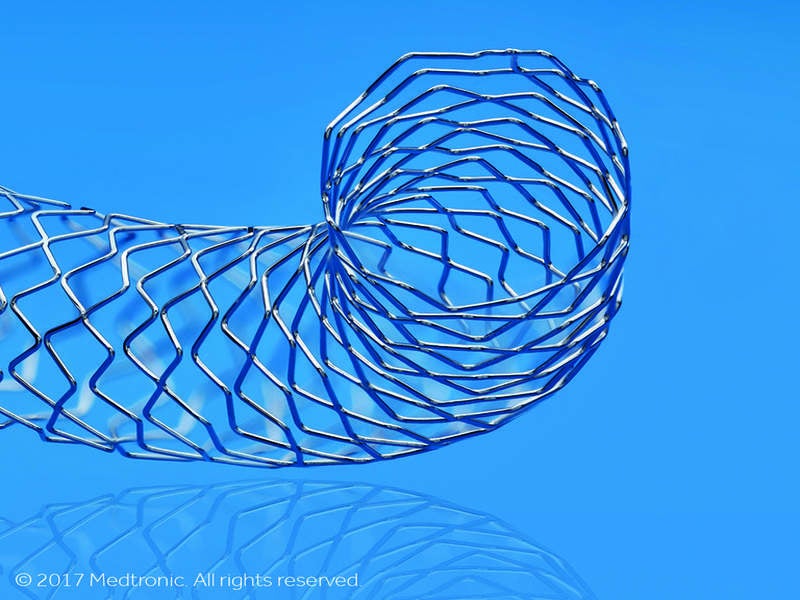
It features core wire technology, which is an upgraded version of the firm’s continuous sinusoid technology method for manufacturing stents that involves the formation of a single strand of cobalt alloy wire into a sinus.
Core Wire Technology enables incorporation of a radiopaque inner core within the cobalt alloy wire for better visibility, and thinner struts to maintain structural strength.
Medtronic Coronary and Renal Denervation business vice-president and general manager Jason Weidman said: “We set out to enhance clinical performance and deliver further meaningful innovations that address the needs of interventional cardiologists around the globe.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The Resolute Onyx DES exemplifies our commitment to advancing our DES portfolio by combining cutting-edge engineering with proven components for a wide range of patients.”
The FDA approval is based on the Resolute Onyx Core (2.25mm-4.0mm) clinical study, as well as the long-term safety and efficacy data that showed low stent thrombosis rates.
The Resolute Onyx DES also includes the BioLinx polymer with a hydrophilic and hydrophobic blend to facilitate endothelial healing, reduce inflammation and lower the risk of stent thrombosis.
Image: Resolute Onyx DES. Photo: courtesy of Medtronic.





