This approval will allow Perosphere to start marketing its products in the EU and other CE mark-accepting countries.
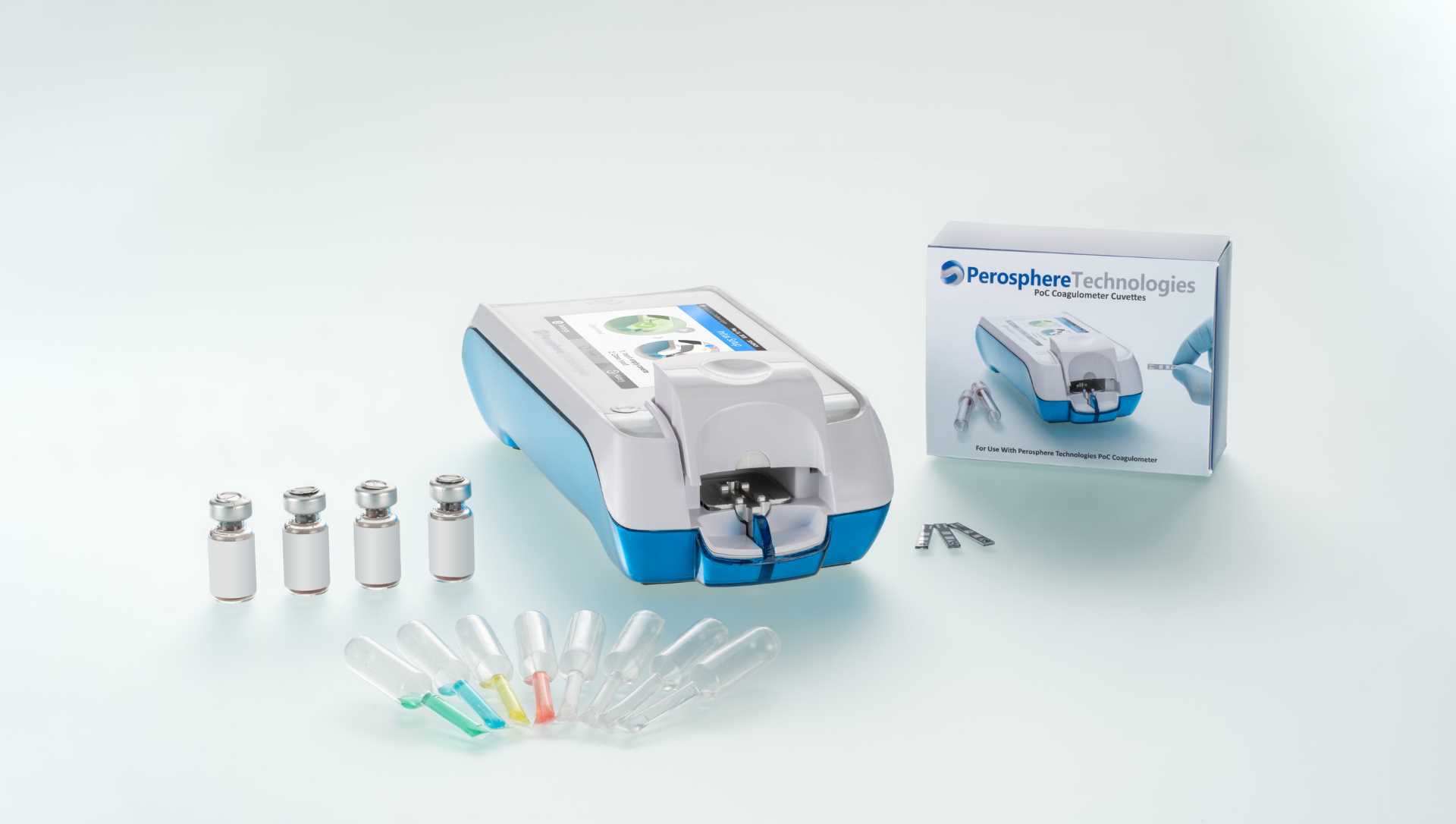
The PoC Coagulometer is a handheld device for use at the bedside of the patient. It has a colour touch screen, barcode reader and eight-hour run-time battery.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The coagulometer technology allows examination of blood coagulability by analysing the clotting time of fresh whole blood, which will meet various unmet medical needs.
Furthermore, Perosphere noted that the PoC Coagulometer is the first device to be marketed for DOAC such as rivaroxaban and low molecular weight heparin (LWMH) coagulation testing in emergency as well as outpatient settings.
The testing with the PoC Coagulometer requires 14µl of fresh venous whole blood, which can be loaded into a single-use cuvette. The result is delivered in four to six minutes, with the whole blood clotting time (WBCT) reported as a clotting time in seconds.
The testing will be able to help analyse if a patient is anticoagulated properly.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPerosphere Technologies CEO Dr Sasha Bakhru said: “We are excited to offer healthcare providers in the EU and other international markets a first-in-class solution for DOAC and LWMH coagulation testing at the point of care.
“We believe our PoC Coagulometer system will change the paradigm for managing DOAC and heparin therapy in emergency settings and beyond.”
Perosphere Technologies intends to introduce the PoC Coagulometer system in some EU countries this year, while an extended launch is anticipated in the first quarter of next year.
Globally, around one million DOAC patients are admitted to the emergency department with severe bleeding such as gastrointestinal bleeding or intracranial haemorrhage, which require immediate surgery, every year.





