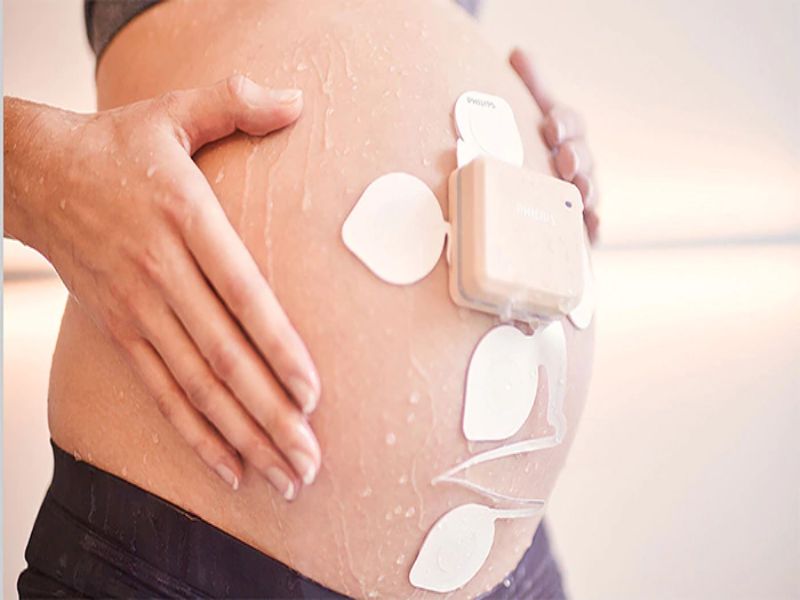
Royal Philips has launched Avalon CL Fetal and Maternal Pod and Patch, an obstetrics monitoring solution to support expectant mothers and clinicians during Covid-19 pandemic.
The high-risk pregnancy solution includes Philips perinatal analytics and visualisation software along with an ultra-portable battery-operated fetal monitor.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Using a single-use, 48-hour, disposable electrode patch placed on the mother’s abdomen, the solution offers continuous, non-invasive monitoring of maternal heart rate, fetal heart rate and uterine activity.
The patch is designed to be placed on the patient by a clinician only once and it does not require frequent repositioning compared to traditional elastic belts and sensors.
Philips Monitoring & Analytics general manager Peter Ziese said: “Remote monitoring during labour has always provided multiple benefits to expectant mothers, including comfort, mobility and flexibility. But during the Covid-19 pandemic, the need for mobile solutions during pregnancy is greater than ever.
“Philips has been dedicated to providing the best quality care for expectant mothers for more than fifty years. This new solution builds on our commitment to provide integrated continuous monitoring capabilities for high-risk pregnancies. With this new patch, clinicians now have access to an innovative tool to help monitor pregnant women during Covid-19, helping to deliver comfort to these mothers during a particularly stressful time.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Avalon Fetal and Maternal Pod and Patch received CE Mark clearance last year and is currently available in select EU countries, Australia, New Zealand and Singapore.
It is the latest addition to the Philip’s remote patient monitoring suite, supporting at-risk populations during the Covid-19 emergency.
Last month, the company acquired 510(k) clearance from the US Food and Drug Administration (FDA) to commercially launch its ultrasound solutions for the management of lung and cardiac complications related to Covid-19.
Philips obtained clearance from Japan’s healthcare authority last week, to launch its Lumify with Reacts handheld tele-ultrasound solution in the country.





