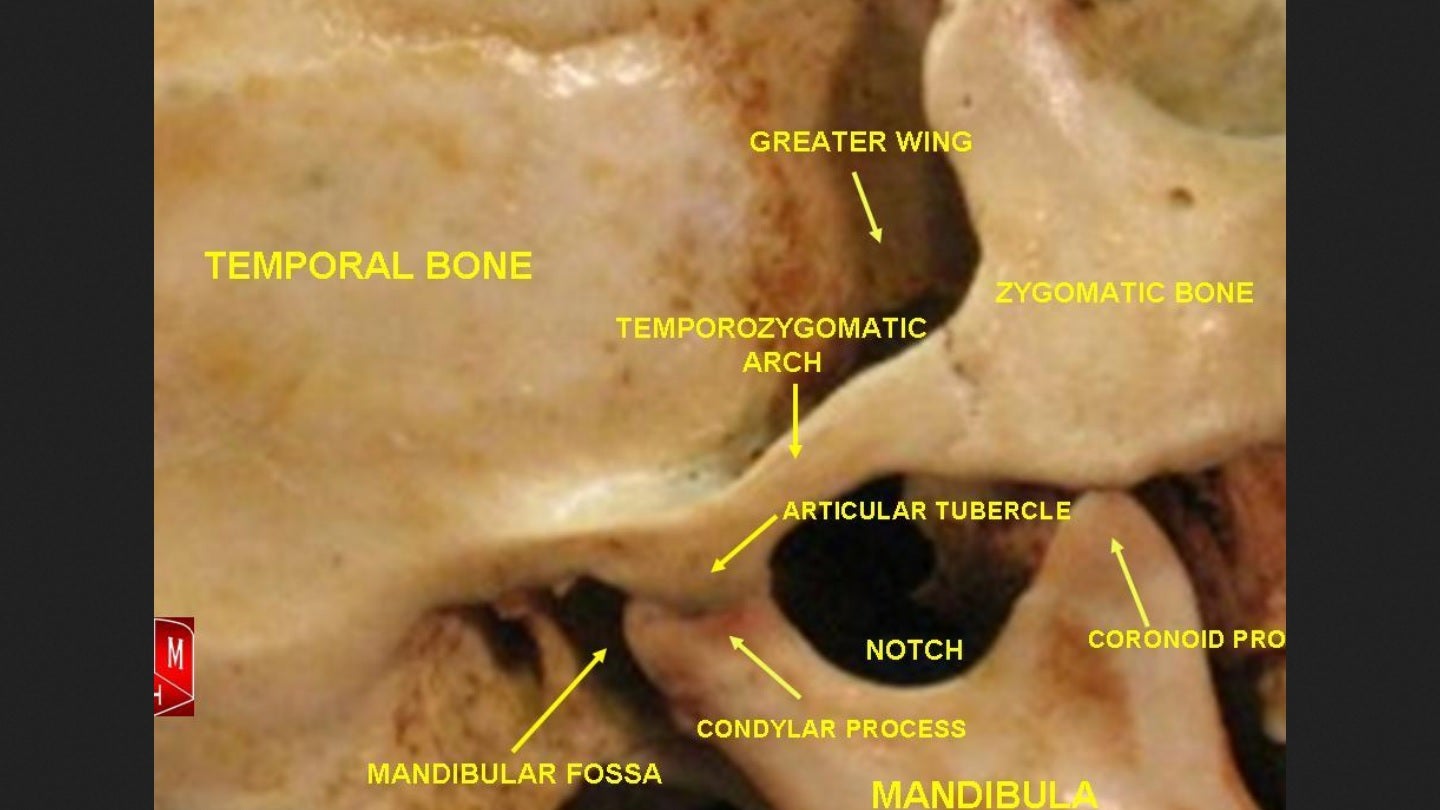
Regenative Labs (Regenative) has announced a new independent study on the application of Wharton’s Jelly for tissue defects associated with temporomandibular disorder (TMD).
The Pain and Sleep Therapy Center, a Regenative observational study clinical site, is sponsoring the study, which has been approved by the Institutional Review Board (IRB) of the Institute of Cellular and Regenerative Medicine.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The single-site, prospective cohort trial will be led by Robinson and NP Rachel Reynolds. Reynolds said: “Working with Wharton’s jelly has opened my eyes to the future for regenerative applications.
“We are so excited to have obtained IRB approval to not only help strengthen the growing body of evidence for Wharton’s jelly application but to also help the population of TMJ dysfunction sufferers who deserve more alternative interventions.”
The objective of this study is to assess the benefits of utilising Wharton’s jelly tissue allografts to address cartilage defects occurring in the temporomandibular joint (TMJ), as well as the potential impact on mastication and TMJ translation.
It will assess the safety of utilising Wharton’s jelly tissue allografts as an alternative treatment option to surgery or other invasive procedures in cases of treatment-resistant TMD.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFurthermore, the study will also evaluate the efficacy and overall improvement of the patients in pain-related symptoms pertaining to bite force tolerance with mastication and lateral translation.
Dr Ryan Robinson said: “We are excited to be pioneers in bringing regenerative medicine into the TMJ world and create an advanced, progressive model for our patients.”





