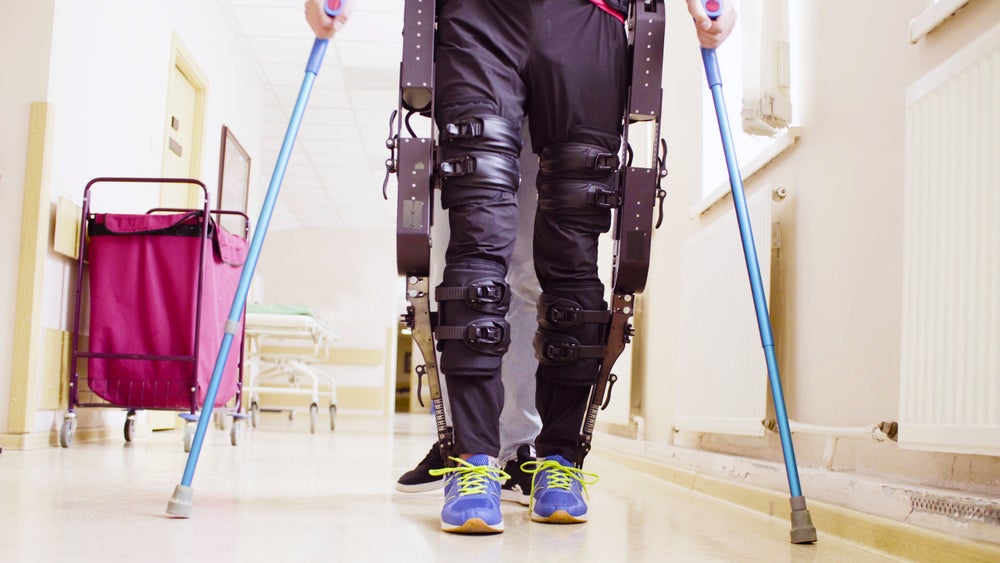
ReWalk Robotics will unveil its exoskeleton at the upcoming Abilities Expo in New York May 5th to May 7th. The technology can help individuals with spinal cord injury access environments otherwise inaccessible due to stairs or curbs.
In March 2023, the U.S. Food and Drug Administration (FDA) granted clearance for the exoskeleton for use on stairs and curbs. This made it the first personal exoskeleton in the US to receive FDA backing for this kind of use.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The expanded feature, which enables spinal cord injury patients to access a wider range of environments, has already been available for some years to patients with paralysis in Europe since its initial CE clearance. Data over 18,000 stair steps from 47 users in Europe was used as support for the FDA submission.
“We are excited to showcase the life-changing functionality and access that our stairs technology provides those with a ReWalk Personal Exoskeleton,” said Larry Jasinski, ReWalk CEO.
“This is truly a first-of-its-kind development, and we are pleased to have actual ReWalk users available to demonstrate not only how this technology works, but also what it means for them in terms of increasing their freedom of mobility and access in their everyday lives.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData




