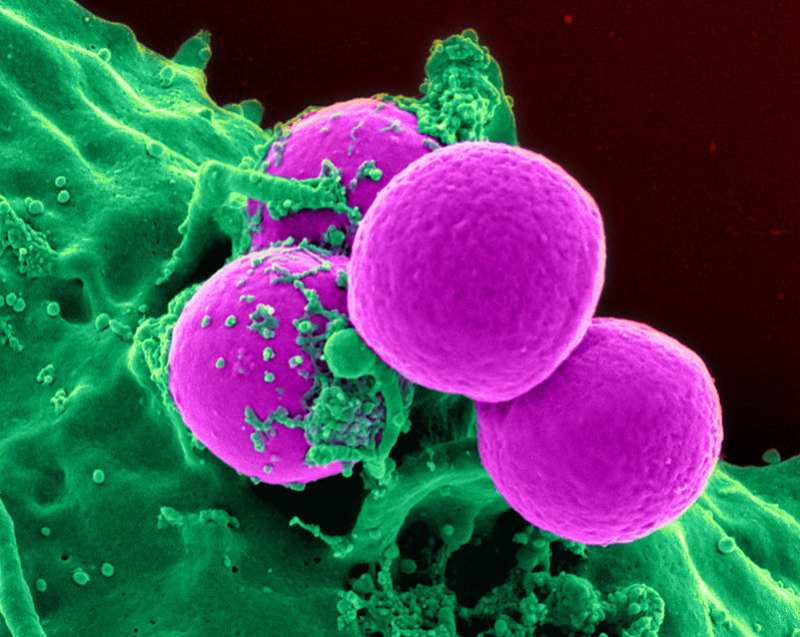
The US Food and Drug Administration (FDA) has granted marketing approval for Roche Molecular Systems’ cobas vivoDx MRSA, a diagnostic test that detects colonisation of Methicillin-resistant Staphylococcus aureus (MRSA) bacteria.
In contrast to conventional culture-based techniques, the cobas vivoDx MRSA diagnostic test uses new technology for rapid detection of bacterial colonisation of MRSA, a common cause of hospital-acquired infections.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
FDA’s Center for Devices and Radiological Health Office of In-vitro Diagnostics and Radiological Health director Tim Stenzel said: “Diagnostics that are able to provide accurate results more quickly can offer health care providers an advantage when trying to prevent and contain the spread of resistant bacteria.
“Today’s authorisation adds a new tool in the fight to prevent and control MRSA in high-risk settings. The FDA remains committed to supporting efforts to address antimicrobial resistance in order to better protect patients against this ongoing public health challenge.”
In the US, nearly 5% of hospitalised patients carry the MRSA bacteria, according to the Centres for Disease Control and Prevention (CDC).
CDC considers MRSA as a severe antimicrobial-resistant threat. If infections develop, they can be challenging to treat as they are resistant to conventional antibiotics.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to the CDC estimates, more than 323,000 MRSA cases in hospitalised patients, as well as 10,000 deaths in the US.
cobas vivoDx MRSA leverages bacteriophage technology based on bioluminescence to evaluate MRSA from nasal swab samples.
The test evaluates the MRSA bacterial colonisation within five hours, compared to two days through the conventional culture method.
Designed to aid in the prevention and control of hospital-acquired MRSA infections, the cobas vivoDx MRSA test will be to identify patients who require enhanced precautions and decolonisation efforts.
The FDA reviewed the test through the de novo premarket review pathway.





