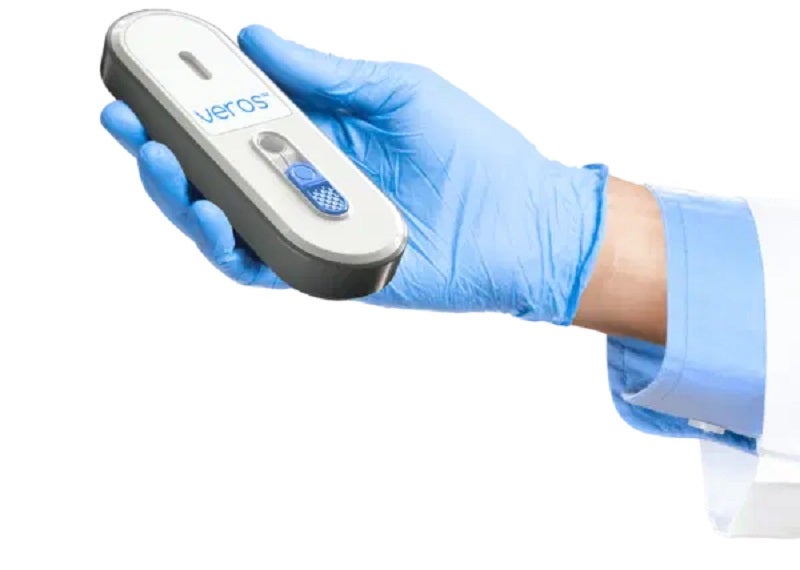
Sense Biodetection has signed a strategic agreement with Abacus dx for the non-exclusive distribution of its Veros instrument-free, point-of-care molecular Covid-19 test in Australia and New Zealand.
Claimed to be the first and only self-contained, single-use Covid-19 product, Veros produces PCR-quality molecular results in around 15 minutes without the need for an external power supply or reader.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It uses rapid molecular amplification technology and aims to improve access to quick, accurate, point-of-care testing.
The Veros test also helps to reduce the spread of Covid-19 through a quicker and more precise diagnosis of the disease.
The assay is able to detect all variants of concern, including all Omicron sub-variants, that the World Health Organization (WHO) and US Centers for Disease Control and Prevention (CDC) have identified up to now.
Under the deal terms, Abacus dx will immediately start supplying the Veros Covid-19 test in Australia and New Zealand after receiving regulatory approval in the countries.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSense Biodetection chief commercial officer Ryan Roberts said: “We are enthusiastic about our partnership with Abacus dx to bring Veros Covid-19 to Australian and New Zealand healthcare providers.
“Together we’re optimistic that by adding the instrument-free Veros Covid-19 to their portfolio, Abacus dx’s customers will be able to take advantage of a rapid, laboratory-quality molecular test that can enable improved access and faster diagnosis, helping them to provide exceptional care for their patients right at the point of use.”
The company received CE Mark for the Veros Covid-19 molecular diagnostic test in March.





