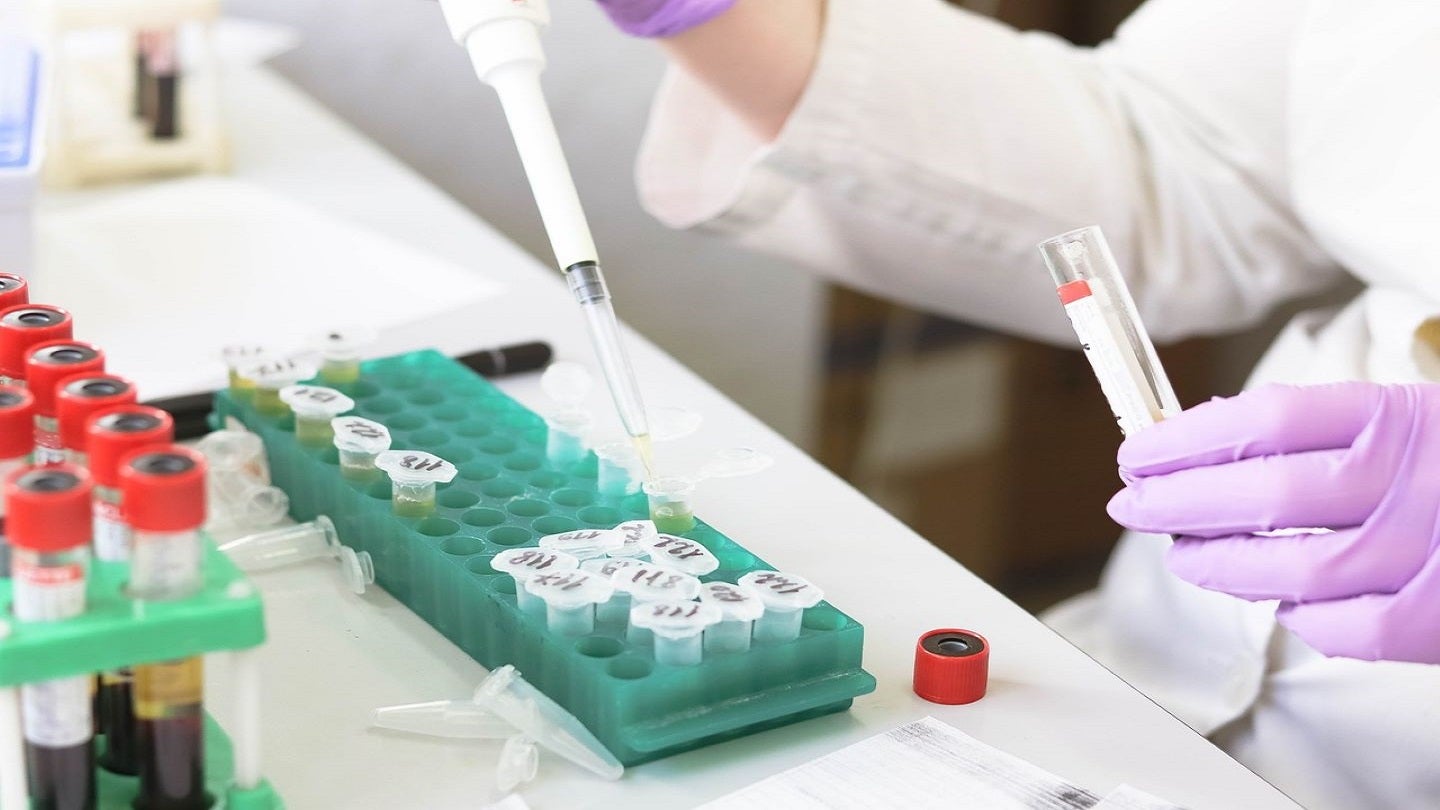
Tempus has entered a collaboration with TScan Therapeutics for the development of a new companion diagnostic (CDx) test.
The partnership helps TScan in implementing its screening protocol for a Phase I clinical trial on solid tumours.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This trial is designed for the administering of customised mixtures of TCR-Ts to patients based on tumour antigen positivity and intact HLA expression.
As part of the collaboration, Tempus’ 648-gene panel, xT assay, will be used for prospectively detecting patients with HLA loss in the tumour to select TCR-Ts, which recognise HLA genes still intact in the tumour of patients.
TScan intends to recruit individuals with solid tumours, including non-small cell lung cancer, melanoma, head and neck cancer, ovarian cancer and cervical cancer, in the trial.
TScan chief medical officer Debora Barton said: “Utilising the assay developed in collaboration with Tempus will help determine if the clinical trial participants’ tumours have undergone partial HLA loss and so will enable us to choose the most appropriate TCR-Ts that are customised for the patient’s tumour antigens and preserved HLA genes.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTempus executive vice-president Michael Yasiejko said: “This CDx work is unique because we’re looking for information that’s not currently in the list of readouts you typically receive from next-generation sequencing of a solid tumour.”
Earlier this year in May, Tempus obtained premarket approval from the US Food and Drug Administration for its qualitative next-generation sequencing-based in vitro diagnostic device, xT CDx.





