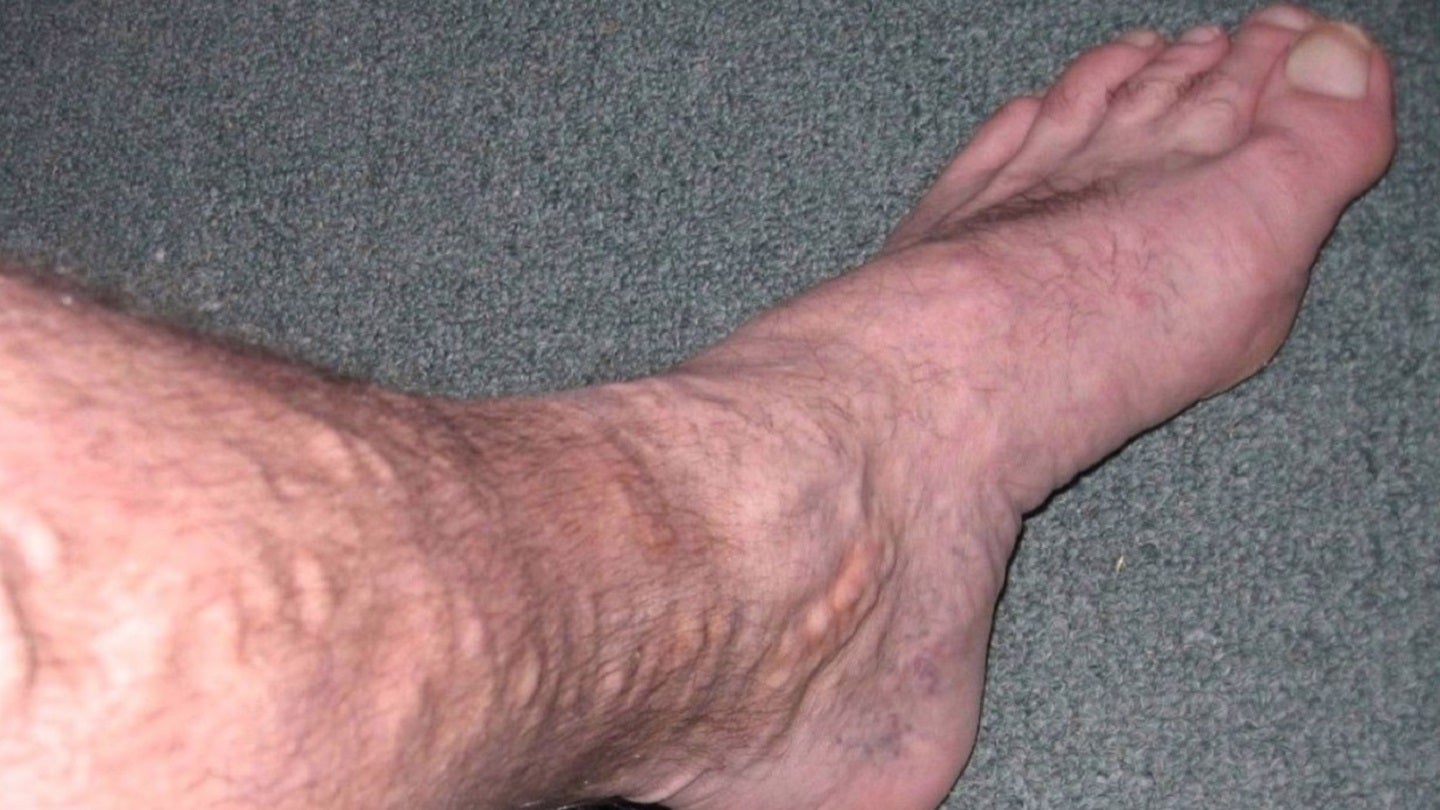
French MedTech company Theraclion has received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to start varicose veins study with its Sonovein device.
Dubbed VEINRESET, the multi-centre pivotal study will assess the efficiency of Sonovein to treat primary insufficiency of great saphenous veins.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Sonovein is claimed to be the first and only robotic solution for the non-invasive ecotherapy treatment for varicose veins.
The CE-marked robotic solution is designed for operation by a single person in a non-sterile environment. Furthermore, it helps to avoid the use of additional accessories, wake-up rooms or hospital beds.
The company will carry out the pivotal study in four centres in the US as well as in Europe.
Englewood Hospital’s Center for Vein Disease director Steven Elias has been appointed to serve as the principal US investigator for the study.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company plans to start treatment of patients in the trial later this year.
Theraclion executive chairman Yann Duchesne said: “We believe that this key study will confirm the positive findings of the FDA feasibility study, completed just two months ago and will ultimately allow us to commercially address the US market.”
In January this year, the company revealed the results of the first Sonovein trial in the US. The study achieved a 100% success rate in the primary endpoint. Venous reflux was eliminated in 95% of the cases.





