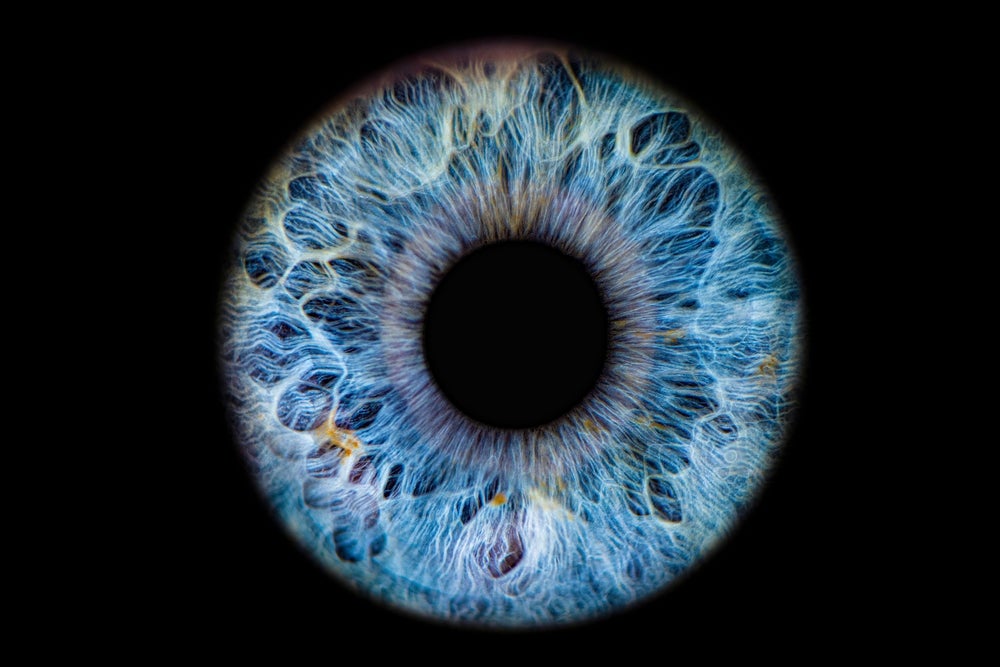
The US Food and Drug Administration (FDA) has granted breakthrough device designation to Toku’s CLAIiR platform, which is an AI-driven software that uses retinal images to assess cardiovascular disease (CVD).
Extensive research suggests the eye could provide a window to the heart. Currently, there are no FDA-cleared or approved devices that use retinal scans to indicate CVD.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
New Zealand-based Toku states its technology is able to provide real-time CVD assessments with similar accuracy to traditional tools such as blood tests, as per a 2 November press release. The AI software detects small details of imaged blood vessels and produces results indicating CVD risk, which are then passed on to the patient’s primary care physician.
Toku added that its software is designed to be integrated into routine fundus retinal imaging cameras.
An estimated 17.9 million people each year globally die from CVD, according to the World Health Organization.
The FDA’s breakthrough device status gives manufacturers prioritised submission review and enhanced feedback from the agency during development.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataToku stated it plans to launch the technology in the US by mid-2025.
Toku co-founder and CEO Ehsan Vaghefi said: “This designation greatly de-risks our clinical development and regulatory pathway for the technology.”
The FDA has already cleared several technologies that indicate diabetes from retinal image scans. LumineticsCore, formerly IDx-DR, launched its FDA-approved AI diagnostic system in the south-east of the US. Its platform detects diabetic retinopathy by analysing retinal scans. The FDA cleared AEYE Health’s AI-powered device and expanded approval of Eyenuk’s EyeArt AI system for the same indication.





