
The use of paper for manufacturer’s instructions for use (IFU) have always been the norm in the medical devices industry. However, thanks to the countless advantages that eIFU (electronic distribution of IFU) brings, more and more manufacturers are now investigating the possibility of replacing their paper IFU by eIFU. But how does that affect their quality management system (QMS)?
The shift towards eIFU is logical considering the numerous advantages it brings. Digitizing the IFU reduces the economic and environmental costs. Restrictions to limit the size of the IFU and cost-of-colour printing no longer applies, so eIFU allows more user-friendly and more legible fonts and improved layouts. Therefore, readers of an eIFU enjoy an improved customer experience. Moreover, eIFU facilitates the process in case of IFU updates. This is because the update only needs to happen online instead of having to replace IFUs in all the boxes you have left in stock.
Nonetheless, eIFU requests some effort to make it compatible with your QMS.
Validation system
First of all, you need a compliant platform to share your eIFU with the customers. The most user-friendly solution is to share the documents over the internet. The customer would visit a given website and would be able to download their documents online. That sounds easy, but you must ensure that the web platform is fully validated by asking yourself the following questions. Does the website employ proper safety standards to protect from tampering? Is the website protected against hardware and software intrusion? Do you have a back-up in place in case someone is not able to access the website or cannot find the IFU online?
These are just a few considerations to be made, which should all be addressed in the validation records to prove the compliance of your system. Moreover, as software gets outdated quickly, it is important to regularly update the eIFU platform under strict change control procedures.
Risk assessment
Second, it is important to perform an in-depth risk assessment. Besides the basic risks associated with your products, you must consider the additional risks associated with eIFU compared to paper versions. Take into account the use of the product, the environment of use, and other end-user’s needs. Consider how patient safety can be ensured when clear working instructions are not provided with the product.
Contrary to the expectations, for many medical devices (MD) and in vitro diagnostics (IVD), eIFU actually have characteristics that help to improve patient safety. For example, legibility is improved in an eIFU, and the absence of constraints on the volume of information provided, reduces the occurrence of user error. The electronic format also makes it possible to add regular, instant updates to the instructions, which reduces the risk of users getting outdated or incorrect information.
As you are obliged to indicate clearly how eIFU should be obtained, users have immediate access to the right document at any time, which eliminates the risk of a paper document getting lost once the package is open.
While all of this yields towards eIFU, it is still important to include the information above in the formal risk assessment. You must provide these rationales to prove you made a well-informed decision.
Update labels
Manufacturers that use eIFU must inform the user on how to obtain the instructions for their products. It is recommended to do so via the product’s labelling.
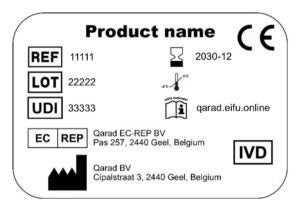
Clear instructions must be provided on how to obtain the respective eIFU, including a unique reference to identify the correct version of the document. This identifier must be defined by the manufacturer and could be the reference number, unique device identification (UDI), Global Trade Item Number (GTIN) or any other unique identification assigned to the product. Moreover, whenever the eIFU has been revised, a clear indication must also be added to alert the customer that the document has been changed.
Last but not least, a customer must be provided with the option to obtain the IFU on paper, upon request, as alternative for the eIFU. The necessary information on how to request that paper copy should be included on the label as well or communicated by other means.
An example of these labelling features is below.
Impact on processes & reflection thereof in procedures
The implementation of an eIFU solution will have an impact on procedures and instructions in the management of eIFU and label content, the inventory management of labelling, the packaging process, logistical process, and the enterprise resource planning (ERP) system. It will also trigger the creation of new procedures for the management of the web platform itself. Although this will initially take some effort, it will considerably reduce the workload of these processes.
Notified Body audit
As the medical devices and IVD industry is highly controlled, the notified bodies will verify all the aspects mentioned above, as well as whether the eIFU solution has been properly validated, whether risk assessment has been performed, quality processes and operational instructions have been adapted, and whether labelling has been modified.
For successful implementation of eIFU, ensure these steps have been addressed.
Conclusion
For quite a long time, the use of paper for manufacturer’s instructions for use were the norm in the medical devices industry. However, many manufacturers are now investigating the possibility of replacing the paper IFU with the eIFU, to gain the benefits of providing this information online. This web-based solution has many advantages, but it has a major impact on the manufacturer’s QMS. As such, it is worth considering outsourcing the project to an experienced partner as it greatly reduces the efforts for the implementation of an eIFU solution.


