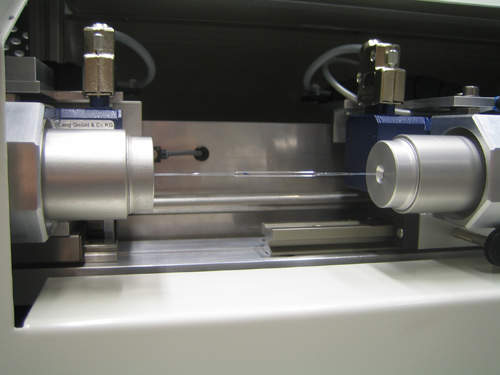
IMSTec offers integrated and innovative manufacturing solutions, including semi-automated processes, complete automated manufacturing lines and manufacturing execution systems (MES), for all types of medical products, including stents, catheters, orthopaedic and pacemaker components, and computer tomography components, tubes or electronic boards.
In cooperation with our customers we accomplish optimised manufacturing targets for quality and productivity based on our standardised products and customised solutions.
Custom manufacturing systems for medical devices
Unique optimised manufacturing solutions require a very structured and standardised development process, starting with a detailed requirement analysis followed by feasibility studies and finally process development and implementation.
Competitive advantages for our customers’ processes can be achieved with an integrated approach taking into account quality, productivity, process and logistics needs.
This joint approach results in innovative solutions, for example packaging, assembly or process lines.
Standardised automation platform for medical device manufacturing
To integrate existing or new customer processes, IMSTec offers a standardised automation platform. The flexible manufacturing cells with integrated processes support easy interchange of individual cells to quickly respond to changing customer needs.
The platform offers automated material handling, and includes a stocker, tray handling equipment and part handling equipment.
Within the integrated micro environment even high-quality clean room classes can be provided (down to clean room class 100). This eliminates the need for external clean rooms.
Automated processes for medical device manufacturing
Depending on the customer’s needs existing processes can be integrated into the automation environment. Alternatively, IMSTec develops automated processes based on product specifications and requirements:
- Inspection (labels, stents, catheters, orthopaedic and pacemaker components)
- Measurement (critical dimension, stent geometry, run out, flatness and alignment)
- Assembly
- Heat treatment (vacuum annealing)
- Coating (drugs and polymers)
- Forming (metal)
- Cleaning
- Laser welding and soldering
- Bonding and curing
- Labelling (laser marking and ink jet marking)
Traceability systems for medical parts
IMSTec’s traceability systems for medical parts are used to support manual, semi-automated and fully automated customer processes for single part and lot traceability. With our forced logistical control system 100% single part traceability is guaranteed, and mixing of parts is prevented.
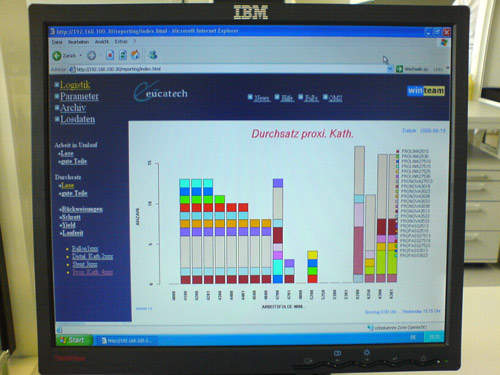
MES software for medical manufacturing
The IMSTec WinTeam MES leads to paperless manufacturing sequence flows. It guarantees continuous optimisation for throughput and cycle time. The WinTeam software is built in a modular way and consists of the following components:
- Access right management
- Production control
- Resource management
- Process management
- Reporting
Automated medical device manufacturing systems
Together with our customers we develop process engineering and translate it into optimum automated manufacturing solutions and systems. IMSTec is DIN EN 9000:2008 certified. The quality standards we meet are CE guidelines, DQ/IQ/OQ documentation, and 21 CFR 820 (GMP) FDA.
Examples of IMSTec’s integrated medical manufacturing solutions include:
- Automated stent manufacturing line
- Automated stent coating line
- Laser welding stations
- Assembly lines: incoming parts screening, plasma activation, merge operations, bonding, label inspection, final surface inspection, critical dimension measurement, screw tightening process and cleaning
- Final inspection systems: stent inspection and measurement system, automated cross-sectioning inspection, automated balloon catheter measurement system and automated electronic board inspection
- Automated metrology systems: alignment / test, form thickness measurement, geometry measurement (stents and catheters), wall thickness measurement (balloon catheters) and profile measurement