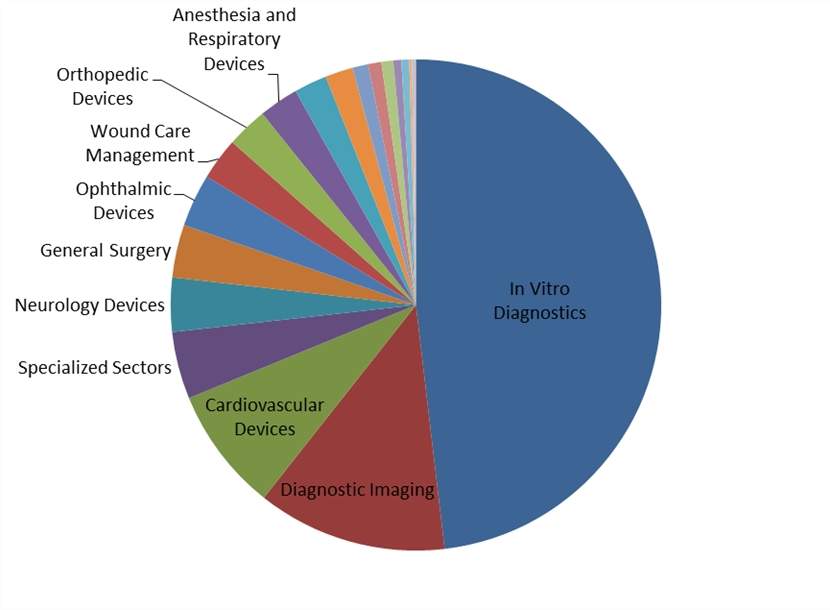
At the outset of 2018, analysis of medical technology pipeline products shows a dynamic market, with particularly heavy investment into in vitro diagnostics (IVD). Almost half of all pipeline products currently in development, excluding those inactive for three or more years, are for the IVD market, as shown in the figure below.
Figure 1: pipeline products by market

Source: GlobalData
The large number of pipeline products in IVD is not unexpected. Many biomarkers have multiple applications, each of which requires separate approval. Additionally, the IVD market spans almost all applications, which greatly increases the potential market size and incentivizes R&D investment.
In recent years, a heavy focus of pipeline products has been in cancer diagnostics. Of the top ten applications for pipeline products, three are specifically for the diagnosis of cancer.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn addition to cancer diagnostics, the third most common application for pipeline products is companion diagnostics. A companion diagnostic test is defined as an in vitro diagnostic device or imaging tool whose use allows the safe application of a therapy. In practice, this involves measuring the expression or presence of a specific biomarker that is linked to a disease condition or therapy to ascertain how a patient will respond to a particular treatment.
Figure 2: pipeline products by application

Source: GlobalData
The increased demand for companion diagnostic tests comes in part from physicians who want to be better informed of the therapy options for their patients, and from healthcare authorities (such as payers) that are looking to control the rising costs of therapies while being able to better identify the most suitable patients for treatment.
However, the greatest driver of increased numbers of pipeline companion diagnostics is the recent changes to FDA regulation of clinical trials. By imposing requirements on correct patient selection, the FDA has effectively made companion diagnostic tests a requirement for any new drug treatment.
In the coming year, the focus on personalised medicine is expected to continue, which GlobalData believes will result in an increasing number of genetic tests and companion diagnostics entering the pipeline.