Bavaria Medizin Technologie to Attend Compamed Fair and MD&M
Bavaria Medizin Technologie has announced it will be attending at this year's Compamed Fair.

Bavaria Medizin Technologie GmbH (BMT) specialises in designing, developing, and manufacturing medical devices.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Bavaria Medizin Technologie GmbH (BMT) specialises in designing, developing, and manufacturing medical devices.
The company’s focuses on catheters, which are used for many different medical applications, from non-vascular ear, nose and throat (ENT) treatments; to the dilatation of arteries; as delivery devices for stents; for drugs or heart valves; and for injection of stem cells into the myocardium.
With more than 25 years’ experience, BMT’s company motto ‘We Know How’ is already well-known in the market and is constantly reflected in the company’s ever-growing global clientele.
BMT performs research and development (R&D) and customised device manufacturing either as a full turnkey solution or as a standalone service.
Tasks are performed quickly and efficiently. The company also provides external services to customers such as biocompatibility testing, accelerated ageing, or pre-clinical testing of devices in animal models. Performed by external partners, these services complete the turnkey solution and make BMT a one-stop-shop for medical devices.
If a customer only requires some services, and not a full package, these can also be ordered as a standalone service, which can be for instance a benchmark testing of a device or the proof of a certain product concept.
The company offers design and development of product packaging, which pass sterilisation requirements and transportation simulation.
Other services available include the crimping of stents onto balloons. BMT has various methods, depending on the stent material and whether it is a drug eluting stent or not. Besides the crimping, BMT also tests the retention force of the stent on the balloon, as well as expansion behavior.
BMT is a pioneer in the development of drug coated balloons (DCB) for coronary and peripheral applications. Its specific clean rooms meet good manufacturing practice (GMP) requirements and the company has an official manufacturing license from the competent authority to handle cytotoxic agents.
The company develops drug coating machines for dilatation balloons and processes according to customers’ needs. BMT also handles the required analytical testing regarding drugs and excipients for the coating formulation and for the finished device, but these tests are carried out at external labs.
As a medical device developer and manufacturer, BMT is certified according to ISO 13485. It also meets regulatory requirements of the ministries of Health, Labour, and Welfare in Japan and the US.
BMT´s quality management system is up-to-date and regularly audited by the company’s notified body and customers. Besides BMT´s own CE approved devices, its regulatory affairs team also assists customers in device approval. This can be as part of an original equipment manufacturer (OEM) / private label manufacturing (PLM) procedure based on BMT´s devices, but also at the end of the device development phase when the design dossier is finalised for the submission to the customer´s notified body. Even if customers are seeking local device approvals in specific countries, BMT offers full support in creating protocols, reports, or other documents, which are required from customers respectively from the local authorities.
BMT is located in Wessling, which is West of Munich, Germany. BMT´s devices and processes are characterised by German engineering, with a very high degree of quality and reliability.
Since 2008, BMT has established a second manufacturing site in Sibiu, Romania, which is also certified according to ISO 13485. With these two manufacturing sites, the company’s production capabilities are very flexible and it is able to manufacture pilot runs and very high volume devices.
So if your requirement is quality, choose BMT: Your partner from concept to market.
Details about BMT’s expertise and services can be found on the Products & Services tab.

Bavaria Medizin Technologie has announced it will be attending at this year's Compamed Fair.

BMT has announced it will exhibit at Compamed Fair in Duesseldorf this year.

With its two manufacturing sites in Germany and Romania, Bavaria Medizin Technologie (BMT) is able to handle pilot run requests and very high volume product demands.
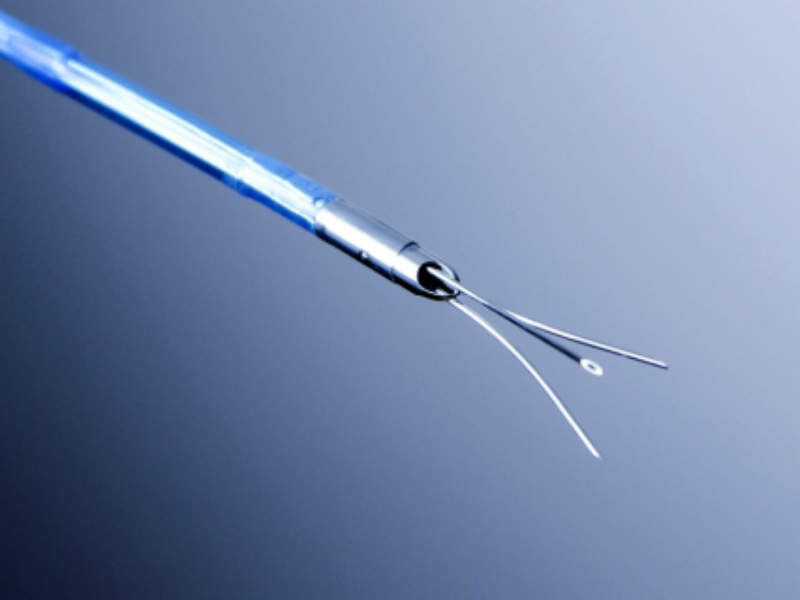
The needle injection catheter (NIC) is a percutaneous device for safe and controlled injection of liquid agents and stem cells into the myocardium.

Although most of Bavaria Medizin Technologie's (BMT) products are made according to customer specification, there are some devices available that are already CE approved by the company and available in its standard configuration.
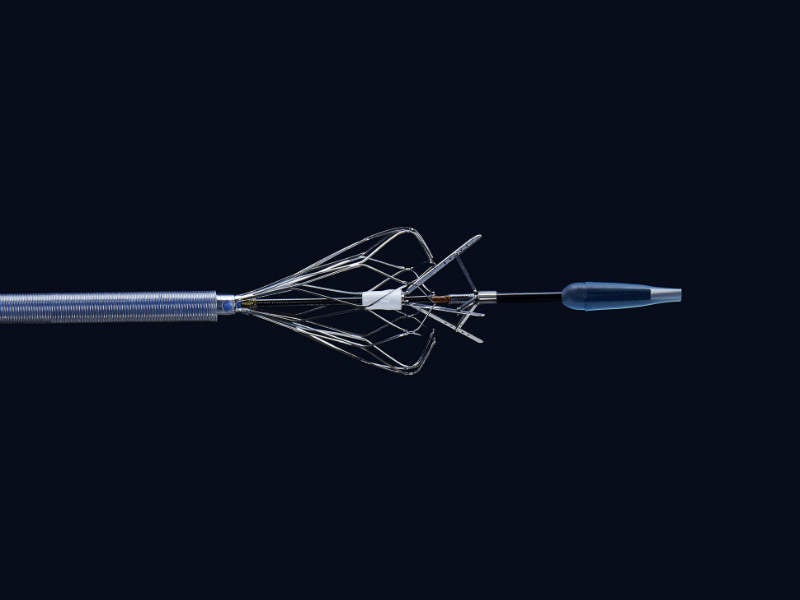
Bavaria Medizin Technologie's (BMT) outstanding development team helps you throughout the entire development process up to regulatory product approval and a transfer into serial manufacturing.
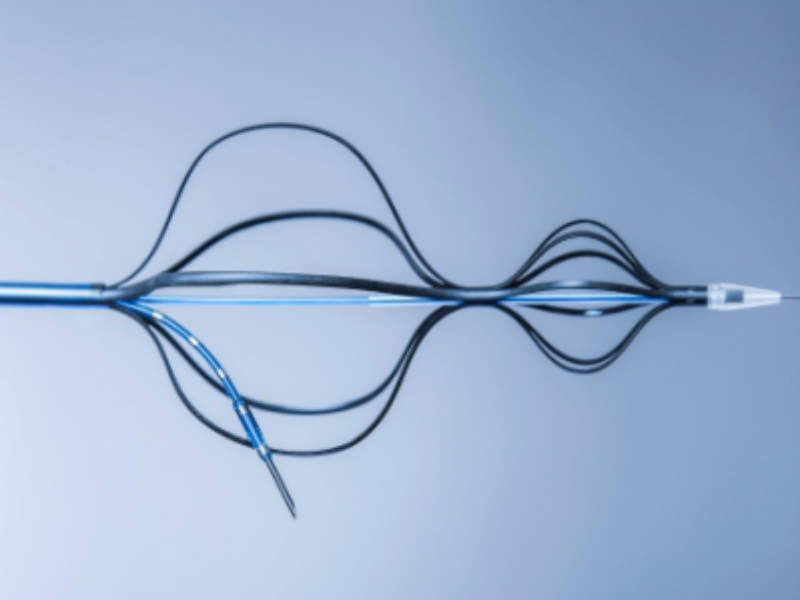
The Kapsus TSP is a mechanical medical device used to provide a secured and controlled puncture from the right atrium (RA) to give other devices access to the left atrium (LA).