Cril - N Tidal B
After winning the "Photonics for Health" Innovate UK competition, Wideblue led a consortium with GSS and CRiL to develop a new range of medical device products - Solid State Optical Respiration Capnometer (SOSORC).

Pivot International (Pivot) provides product development and manufacturing services of medical devices for customers and applications worldwide.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Pivot International (Pivot) provides product development and manufacturing services of medical devices for customers and applications worldwide.
Our flexible and knowledgeable team augments our customers’ in-house capabilities throughout the product lifecycle: concept, development, manufacturing, launch, and sustaining. We also provide complete turnkey projects.
Customers range from new inventors to large, well-established firms, and we work to ensure we have the right combination of development and manufacturing skills to serve them all well.
Pivot’s product development team has decades of experience successfully deploying hardware, software, mechanical, and industrial designs for medical products.
We skillfully integrate the lessons learned from these projects with the latest advances in embedded control, user interface, communications, power management, and motor control to ensure the resultant product is not only fit for purpose, but also reliable, manufacturable, and competitive.
One of Pivot’s core values is flexibility, which is evident in our approach to manufacturing. We design our manufacturing strategy to adapt to our customers and scale as they scale, providing a full range of medical device manufacturing capabilities ranging from printed circuit boards (PCBA) to complete product assembly.
Our global manufacturing footprint comprises six manufacturing facilities in the US, Europe, and the Philippines.
We have over 200,000ft2 of manufacturing space and the ability to efficiently scale from prototype quantities as products are developed and launched to mass production as products succeed in the market.
Our facilities include more than 16,000ft2 of International Organisation of Standardisation (ISO) Class 8 cleanroom environment for general manufacturing and 2,100ft2 of ISO Class 7 cleanroom environment for sterile packaging of medical devices.
Our global footprint gives us and our customers the ability to access the global supply chain. Whether it is components for a PCB, tooling, moulded or cast parts or anything else required by our manufacturing facilities, our supply chain team works to ensure we are sourcing competitively and responsibly.
Recognising the significance of design changes in the regulated medical industry, we also work proactively with our product development team to ensure the most extensive product lifecycles possible and to minimise disruption when changes are required.
Through our client collaborations to bring a broad range of medical and diagnostic devices to market, we gained hands-on experience working within the various regulatory and quality frameworks for these devices.
Our experience includes:
Our innovative applications of infrared, optics, imaging systems, wireless / Internet of Things (IoT), and digitisation in medical applications has earned international recognition for multiple customers and products. Recent awards include:
Pivot International was founded in 1972 and has evolved into one of the fastest-growing privately held companies in the US.
We facilitated this growth by serving our medical customers, as well as success in other markets such as industrial, agriculture, security, and defence.
The breadth of applications we solve in these markets allows us to complement our medical expertise with diverse perspectives and generate winning solutions for all our customers.
Recent recognition of our growth includes Kansas City Business Journal Fast 50 (#8), Ingrams Corporate 100 – Fastest Growing Companies in Kansas City (#11), and Inc 5000 – Fastest growing privately held companies in the United States (#838).

After winning the "Photonics for Health" Innovate UK competition, Wideblue led a consortium with GSS and CRiL to develop a new range of medical device products - Solid State Optical Respiration Capnometer (SOSORC).

Pivot International’s subsidiary, Digital Concepts (DCI), has development expertise in touchscreen control and graphical monitoring technologies and are able to power a family of products (bikes, ellipticals, treadmills, rowers, etc.). They use multi-media TFT displays, custom etched LCD’s, LED displays, NFC, BLE, and in-house designed worldwide TV tuners. They provide a turnkey console solution that also includes injection mould tooling, sheet metal brackets, button/switches, and packaging.

Our Surgical Drill Driver is a battery-powered intelligent drill driver for affixing surgical plates to bone. It is used within the surgical suite for neurosurgical procedures involving drilling and driving titanium and bioresorbable screws.

This medical camera merges data acquisition with storage and management via a web application. It allows physician-to-patient sharing, synchronization of patient folders, and HD image or video transmission by a secured connection to a referring doctor – anytime, anywhere. The camera offers a unique experience with a removable LCD touch screen. This first-to-market concept allows the surgeon to instantly share and review their findings with the patient and colleagues locally or remotely.

This medical camera merges data acquisition with storage and management via a web application. It allows physician-to-patient sharing, synchronization of patient folders, and HD image or video transmission by a secured connection to a referring doctor – anytime, anywhere. The camera offers a unique experience with a removable LCD touch screen. This first-to-market concept allows the surgeon to instantly share and review their findings with the patient and colleagues locally or remotely.

This sensored prescription bottle continuously monitors prescription drug usage to ensure the proper rate of consumption, improving patient outcomes. Monitoring data is directly uploaded to the cloud after each bottle usage, with sensor sensitivity capable of measuring a fraction of a single pill weight. The device’s form factor and appearance are that of a typical prescription bottle, with miniaturized electronics in the base of the bottle supporting >60-day prescription for most pill types.

Peek Vision is a social enterprise and UK registered charity comprising of technologists, eye specialists, and product designers who are passionate about making high quality eye care available to everyone. Peek approached Wideblue, a subsidiary of Pivot International, to develop a low-cost ophthalmoscope to help bring essential eye care to low-income countries such as Tanzania.

Late in 2008, The Impossible Project took over part of the last Polaroid integral instant film manufacturing plant and reinvented a totally new instant film for traditional Polaroid cameras.

Centeo Biosciences conceived the TG40 System, a portable unit that allows scientists to control the temperature of protein crystallisation samples during experimental procedures. The device had to have a high degree of temperature uniformity and setpoint accuracy to ensure optimal crystallisation.
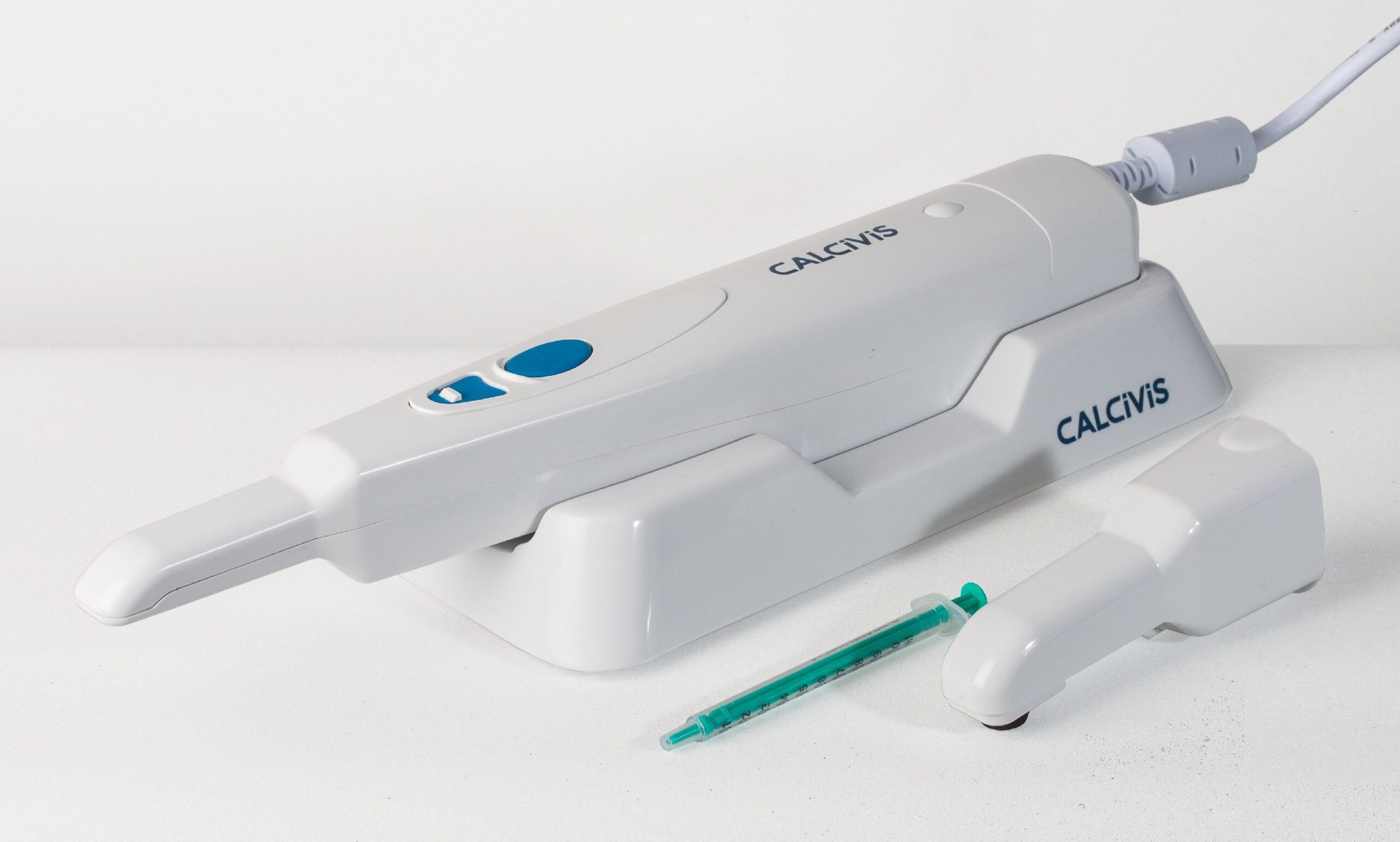
The CALCIVIS imaging device is a specialised device designed exclusively to image the tooth after delivering an application of CALCIVIS photoprotein. The handheld medical device is initiated via a 'one-touch' computer-controlled process. A specialised sensor integrated into the device immediately detects the resulting luminescence (light flash), and in less than one second, bespoke software presents a chairside demineralisation 'hotspot' image map to clinicians, enabling informed and efficient dialogue with patients.

ReproCELL Europe's PM1 instrument provides real-time information on drug potency by measuring changes in the shape and form of isolated blood vessels during a pre-programmed perfusion schedule.

After winning the "Photonics for Health" Innovate UK competition, Wideblue led a consortium with GSS and CRiL to develop a new range of medical device products - Solid State Optical Respiration Capnometer (SOSORC).

As part of a proof-of-concept feasibility study, Wideblue worked with Anasyst and the University of Teesside to design a point-of-care technology demonstrator for the early detection of Sepsis.

When Wisconsin-based WiscMed co-founder and Wispr-inventor Dr James Berbee decided to take on the formidable challenge of pursuing a medical degree from Stanford at age 42, little did he know he’d be behind a medical innovation six years in the making that would advance the standard of paediatric care.