Visit our Covid-19 microsite for the latest coronavirus news, analysis and updates
Follow the latest updates of the outbreak on our timeline.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
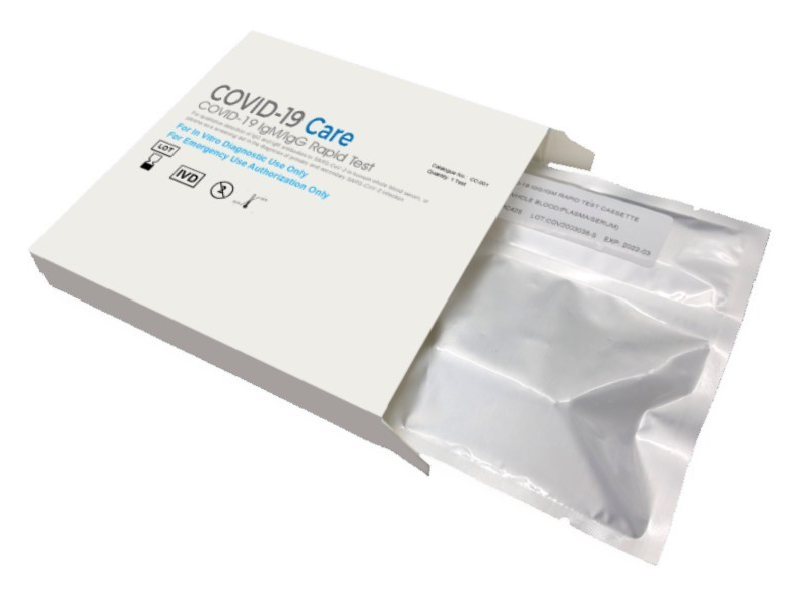
Molecule Holdings has launched its coronavirus (Covid-19) diagnosis test kits for immediate distribution to medical professionals, following its emergency use authorisation (EUA) by the US Food and Drug Administration (FDA).
The Covid Care Rapid test detects IgG and IgM antibodies to SARS-CoV-2 in human blood and delivers results in five minutes. It is said to have a 91% clinical sensitivity rating and a 99% clinical specificity rating.
Furthermore, the test offers affordable, point-of-care testing compared to PCR technology-based coronavirus tests, which take multiple days to show results.
The Covid Care Rapid test was developed by Pinnacle BioLabs. It is manufactured in ISO -13485 certified facility.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPrior to the launch, the test has undergone significant preliminary clinical testing and can provide diagnostic specificity higher than 90%.
The test kit contains a lancet to prick the finger and testing cassette to place blood drops. It also comes with a testing solution, which has to be mixed with the obtained blood to get the result.
The testing process is similar to the tests used by diabetics to test their blood sugar.
Molecule Holdings will soon provide the test kits to medical professionals, first responders and law enforcement.
Meanwhile, medical companies and laboratories across the US are striving to develop and produce more Covid-19 detection tests to meet the increasing demand.
Recently, FDA granted EUA to several companies, including Bodysphere, Luminex, and Abbott for their Covid-19 detection tests.
According to the latest reports, there are more than 216,721 confirmed coronavirus cases and 5,138 deaths in the US.





