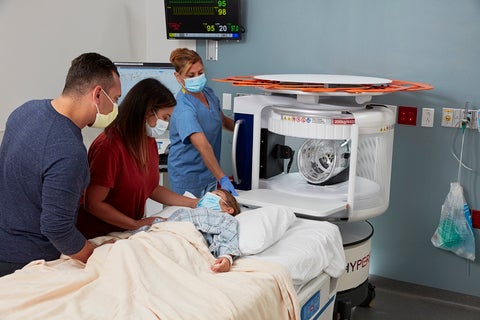
Hyperfine has completed enrollment in its multi-site observational study evaluating the use of its portable low-field magnetic resonance imaging (MRI) system in children with neurological injury.
The pilot study, which is using hydrocephalus as an index condition, will assess if images from the system – called Swoop – can be used in children to help manage the condition.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Children living with hydrocephalus – a build-up of fluid in the brain – undergo regular scans each year to monitor the pressure in the enlarged brain ventricles and identify potential malfunctions in implanted devices used to drain excess fluid, known as a cerebral shunt.
Hyperfine’s US Food and Drug Administration (FDA)-cleared Swoop system can provide brain MRI capabilities at the point of care. It can be used as a bedside device for obtaining brain images when other clinical avenues are not practicable.
“The Swoop system represents a promising shift in brain imaging. We believe it has the potential to improve paediatric hydrocephalus management significantly,” said Dr. Khan Siddiqui, chief medical officer, and chief strategy officer at Hyperfine.
“The insights gathered from this study will inform the viability of the Swoop system in pediatric healthcare settings and patient care.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Connecticut, US-based company recently began a separate mutli-site, observational study assessing the device’s use in ischemic stroke detection.
Since receiving FDA clearance in 2020, the device has been approved for use in the UK, Canada, Australia and, in February 2023, picked up CE marking for use in Europe.
A market model by GlobalData estimated that the global MRI market was worth $5bn in 2022. It is forecast to double, reaching $10bn by 2033.





