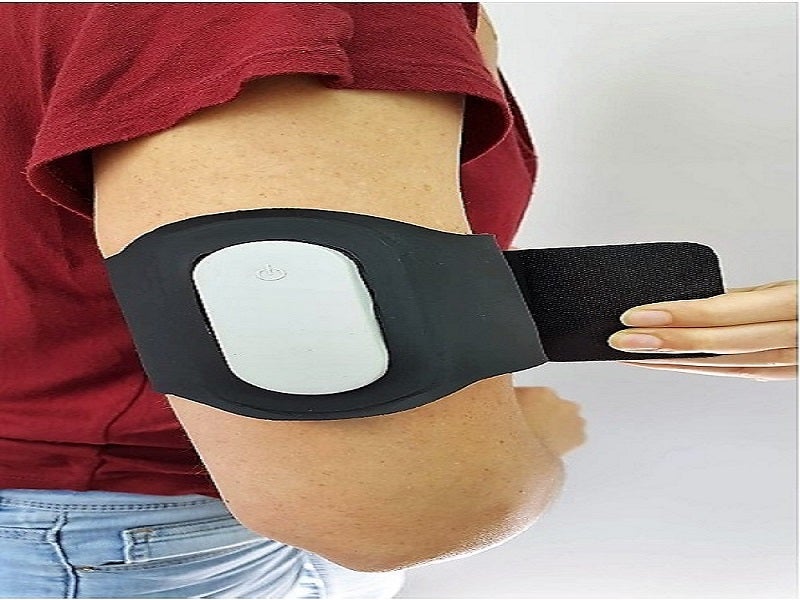
Nerivio® is a smartphone-controlled wearable neuromodulation device developed by Theranica Bioelectronics, a digital therapeutics company based in Israel, for the acute treatment of episodic or chronic migraine in adolescents.
The electroceutical device is intended to provide safe and effective relief from migraine pain without the side effects that are typically seen in therapeutic drug use.
Nerivio can be accessed by any licensed healthcare professional, including telemedicine systems such as Cove and UpScript, with a prescription. It can also be delivered directly to the patient’s home.
Th device is available to veterans through a Federal Supply Schedule (FSS) agreement signed between Theranica and the United States Department of Veterans Affairs in February 2021.
In January 2023, Theranica collaborated with Dr Reddy’s Laboratories, a pharmaceutical company based in India, for the marketing and distribution of Nerivio in India.
Regulatory approvals for Nerivio
In May 2019, the Nerivio device received De Novo approval from the US Food and Drug Administration (FDA) for clinical use. The FDA classified the device as a distal transcutaneous electrical stimulator for the treatment of acute migraine.
In September 2020, Nerivio received a CE mark under the new medical device regulation (MDR) European standard for acute migraine treatment.
In January 2021, the FDA expanded the device’s indication to include the acute treatment of episodic or chronic migraine in patients aged 12 years and older. Nerivio later received FDA clearance as a dual-use acute and preventive treatment for migraines in individuals aged 12 years and older with or without aura in February 2023.
Nerivio’s design and features
Nerivio is a non-invasive, drug-free, easy-to-use electronic device powered by a non-rechargeable battery. The integrated device is worn on the upper arm.
The device uses non-painful remote electrical modulation (REN) to activate peripheral nerves, inducing the internal pain management mechanism known as conditioned pain modulation (CPM) in remote body regions. It resembles a sports armband and operates through a mobile application installed on a smartphone or tablet to control and monitor the treatment and other features.
The device comprises an electronics case, firmware, mobile application software and the armband, which is equipped with electrodes covered with hydrogel.
The plastic electronics case contains an on/off switch and light-emitting diode (LED) indicator, while the mobile application software is installed and run on a smartphone or tablet. The software controls the device and allows data and records to be stored and received.
With its new dual-use indication, Nerivio is effective for 12 to 18 treatments for 45 minutes and can be used more frequently to prevent migraines proactively. Patients can choose the number of refills in the prescription included in the app.
Nerivio’s mechanism of operation
Nerivio transmits weak electrical pulses on the skin for 45 minutes at a time to produce transcutaneous electrical nerve stimulation, generating CPM to inhibit migraine pain.
The treatment intensity is indicated on a scale of 0-100 on the device’s output power. The ABORT button can be pressed to stop the treatment.
A biphasic rectangular waveform is delivered via a single channel at a modulated frequency of between 100Hz and 120Hz, with a 400µs pulse width and an output current of up to 40mA. The patients can self-administer the treatment at the onset of a migraine attack.
Technology used in the device
Nerivio is integrated with state-of-the-art technology, including various neuromodulation and neuroscience advancements. The device’s innovative design produces a patented waveform delivered to C-fibre nerves, which triggers a pain-reducing mechanism of analgesics in the brain stem.
The company uses electrical nerve stimulation technology, neuromuscular electrical stimulation (ENS/NMES), and Bluetooth low energy. It also uses the company’s patent-pending Maximum Effectiveness mechanism to collect and determine electromyography signals from the treated muscle.
The mobile app, when integrated with the Nerivio technology, allows users to tailor their migraine treatments according to patients’ needs and convenience. The software allows them to receive reminders for preventive therapies, track the patient’s migraine patterns, and optionally share migraine data with their doctor. The application also helps users through a Guided Intervention of Education and Relaxation (GIER) using techniques such as diaphragmatic breathing, muscle relaxation, and guided imagery.
Clinical studies on Nerivio
The FDA’s approval of the Nerivio neurostimulation device was based on a prospective randomised, double-blind, sham-controlled, multi-centre clinical trial, in which 296 participants with acute migraine were enrolled. The trial was conducted at seven sites in the US and five sites in Israel.
Patients were randomised in a 1:1 ratio to receive stimulation either via the active or sham device for 30-45 minutes on the upper arm within one hour of the onset of a migraine. The sham device produced stimulation at a lower pulse width and frequency.
The study’s primary endpoint was the percentage of patients with reduced migraine headache after two hours of treatment. Stimulation with the active device was found to be more effective than that with the sham device, with 66.7% of Nerivio patients reporting a reduction in migraine headaches compared with 38.8% of patients receiving stimulation via the sham device.
The active device was therapeutically beneficial to 27.9% of patients, while around 37.4% were pain-free after two hours of treatment. Adverse events related to the device were also low.
Clinical studies for dual-use approval of Nerivio
Theranica conducted a preventive double-blind, randomised, placebo-controlled clinical trial that demonstrated Nerivio’s efficacy and safety when used every other day for the prevention of migraine.
A total of 248 participants were studied in the trial and used either REN with Nerivio or a placebo wearable every other day. The trial comprised a four-week baseline observation phase, followed by an eight-week double-blind intervention phase.
Patients who used Nerivio observed a mean reduction of four migraine days each month from baseline, compared with a reduction of only 1.3 days in the placebo group, with a significant therapeutic gain of 2.7 days a month.
The study also determined the reduction of monthly migraine days separately for chronic migraine participants, who reported a reduction of 4.7 days compared with 1.6 days for placebo, and episodic migraine patients, who reported a reduction of 3.2 days compared with one day for placebo.
The results showed that participants also experienced statistically significant reductions from baseline in the mean number of headache days of all severities, as well as the number of days on which they required acute migraine medication.
These results indicate that REN is a safe and effective preventive treatment for migraine, offering a much-needed non-pharmacological alternative either as a standalone preventive therapy or in combination with pharmacological therapies to improve preventive impact.
The trial data demonstrates that given its previously well-established clinical efficacy and high safety profile in the acute treatment of migraine, REN can cover the entire treatment spectrum, including both acute and preventive treatments.