The Vercise™ Gevia™ deep brain stimulation (DBS) system is a directional neurostimulation device developed by Boston Scientific for the symptomatic treatment of patients with Parkinson’s disease, dystonia and essential tremor.
The device is based on cochlear implant technology, which stimulates auditory nerves, producing a hearing sensation.
Regulatory approvals
The Vercise™ Cartesia™ directional lead was launched first in Europe in 2015 and subsequently in the global market. The Vercise™ Gevia DBS system received a CE mark for marketing in Europe in June 2017.
The first-generation Vercise™ DBS system was approved by the US Food and Drug Administration (FDA) in December 2017. The FDA approved the Vercise™ Gevia™ with Cartesia directional lead for clinical use in January 2019.
In August 2019, the FDA approved the ImageReady™ MRI labelling for the Vercise™ Gevia™ DBS system, which enables patients implanted with the device to safely undergo a full-body magnetic resonance imaging (MRI) scan.
The FDA approved the fourth generation Vercise™ Genus™ DBS system for conditional use in an MRI environment in January 2021. The MR conditional DBS system is customisable for every patient and features an MR conditional portfolio and wireless Bluetooth® connectivity to deliver the next level of precision in directional stimulation.
In April 2022, the FDA granted approval to Boston Scientific’s Vercise™ Neural Navigator with STIMVIEW™ XT, which is a next-generation image-guided programming software for DBS therapy.
The software was developed in collaboration with Brainlab, a software-driven medical technology company. It enables 3D visualisation of both lead placement and stimulation of brain anatomy by clinicians in real-time.
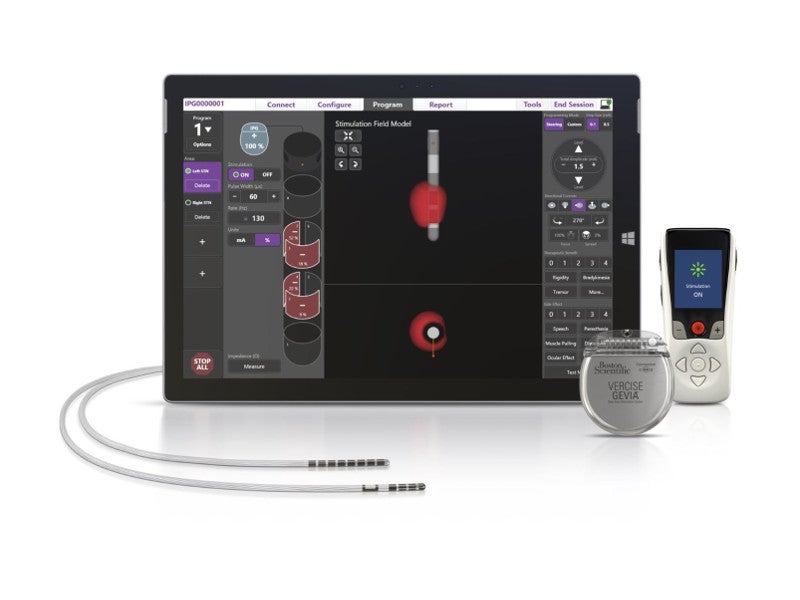
Vercise Gevia deep brain stimulation system design and features
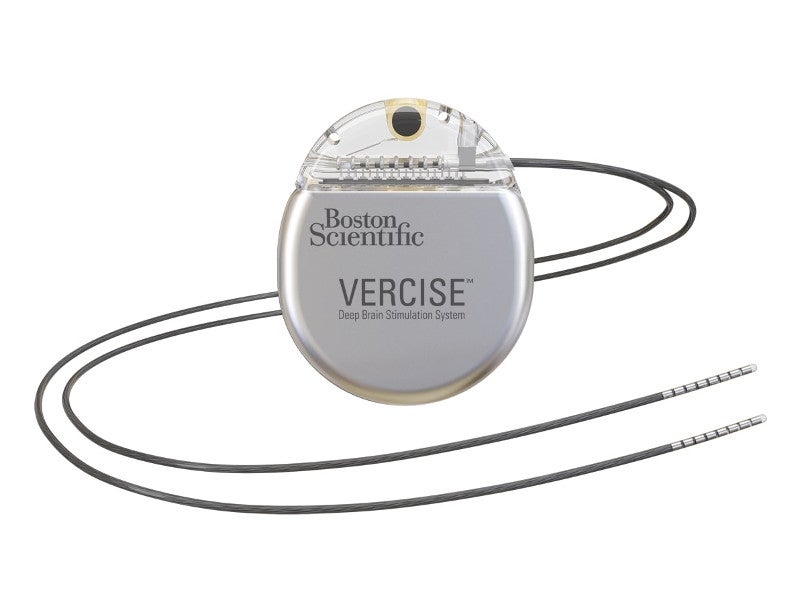
The Vercise™ Gevia™ DBS system is a full-body MR conditional device comprising three components – a non-rechargeable or rechargeable implantable pulse generator (IPG), a patient controller, and a lead kit and extension kit.
Vercise™ Gevia™ works by delivering low-intensity electric impulses through surgically implanted leads in the brain connected to an IPG to reduce signs and symptoms of advanced Parkinson’s disease and essential tremors.
Two Vercise™ Cartesia™ directional leads of the system, containing eight separately controlled cylindrical electrodes, are implanted into the brain. The leads create precisely targeted stimulation at the subthalamic nucleus (STN) in the brain. The STN is adaptive to the changes occurring in the brain. Its simulation provides symptomatic relief and avoids unwanted side effects.
The titanium-cased stimulator is implanted under the skin at the same side of the lead near the pectoral region with an extension connected directly to the stimulator. The rechargeable battery of the system lasts for approximately 15 years. A tunnelling tool is used to make the required path under the skin for implanting the DBS leads and extensions.
The SureTek™ Burr hole cover is used to anchor the leads and is compatible with a 14mm perforator. A radiofrequency telemetry link is used to communicate with the stimulator, using a handheld remote control from a distance of 18in. The remote control can be programmed using the Bionic Navigator™ software.
Before a patient undergoes an MRI scan, the stimulator is fully charged and the MRI mode on the system is enabled. MRI systems with the magnetic strength of 1.5 Tesla (1.5T), maximum spatial field gradient of 4,000 gauss per cm (40T/m), and gradient systems with a maximum gradient slew rate per axis of less than or equal to 200T/m/s are eligible for scanning the patients with the implanted DBS system.
Software details
The neurostimulation system features Vercise™ Neural Navigator 2 programming software with STIMVIEW™ technology.
The software allows healthcare professionals to visualise the distribution of stimulation into the brain. It enables the range, direction, and shape and size of the electrical impulses to be controlled to provide highly personalised therapy to the patients.
Clinical trials on Vercise Gevia deep brain stimulation system
The clinical efficiency of the DBS system was demonstrated in a multi-centre, prospective, double-blind, randomised controlled clinical study named INTREPID. The trial enrolled 292 patients with Parkinson’s disease and demonstrated approximately 48% improvement in the motor skills of the patients treated with the system.
The improvement was measured for more than two years by the Unified Parkinson’s Disease Rating Scale (UPDRS) III scores, which are used to determine the overall motor disability.
In another clinical study, VANTAGE, 40 Parkinson’s patients were treated with the DBS system. At week 52, 63% of the patients showed improvement in motor skills and quality of life.