
Regulatory authorities, such as the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other global regulatory bodies, regularly update their guidelines to align with scientific advancements and enhance patient safety. As a result, the regulatory landscape is constantly evolving to keep up with technological advancements and address potential risks. With so many medical devices in the product pipeline, failure to keep up with these changes could drastically slow down time to market.
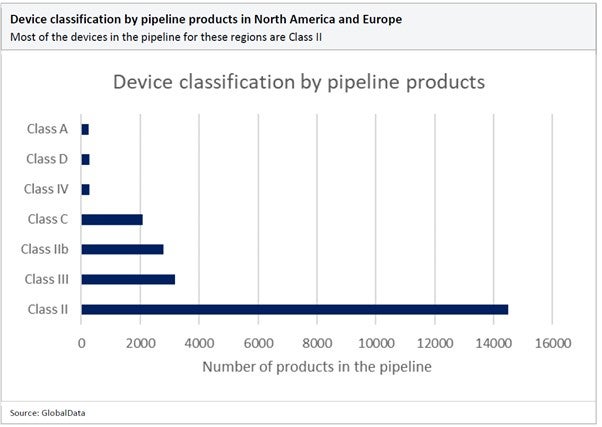
But the onus of dealing with growing regulatory complexities as medical devices become more digitalised and advanced is not entirely on the manufacturers. Regulatory bodies are under pressure to provide guidelines that keep patients safe, but also do not hinder innovation. According to GlobalData’s Medical Device Predictions 2023 report: “At present, regulations are complex and the process is notoriously challenging for developers to pass products through the regulatory framework. In a bid to encourage innovation and ensure delivery of more novel, safe, and effective innovative medical devices to patients, local authorities are encouraged to provide clear and appropriate guidance.”
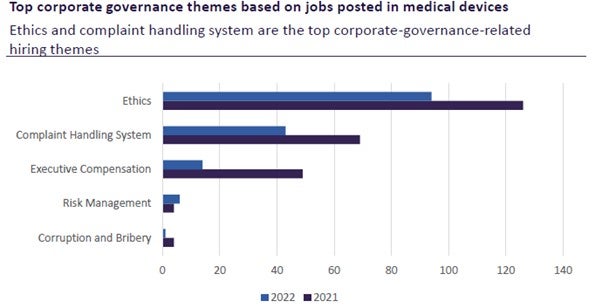
In addition to these evolving regulations, medical device manufacturers must also be aware of their corporate governance performance. GlobalData’s Global Corporate Governance Trends in Healthcare, 2022 report states that “the pharmaceutical, healthcare, and medical devices sectors are all highly regulated, but they still face controversies. Responsible governance is a key requirement for sustainability in the pharma industry. Regulations, external initiatives, and commitments to international guidelines are constantly evolving.”
According to Alexandra Murdoch, GlobalData medical analyst: “While the medical device industry faces stringent regulations, the industry must still acknowledge their roles in social inequity, corruption, tax avoidance, and lack of security.”
Ensuring compliance
Navigating the complexities of the regulatory landscape can be difficult, especially if manufacturers are reaching a global market and dealing with differing product categorisations and standards in each region.
Companies must actively monitor regulatory updates and guidance documents from relevant authorities. This also involves ensuring that all partners involved in manufacturing are compliance informed. After all, a supply chain that lacks transparency could be detrimental to auditing processes.
Implementing a robust quality management system (QMS) aligned with ISO 13485 is essential. A well-defined QMS helps ensure adherence to regulatory requirements throughout the product lifecycle, from design and development to manufacturing and post-market surveillance.
According to Richard Ohidy, quality engineer at the Alleima production unit in Palm Coast, USA: “Regulatory compliance is crucial to every organisation, which must be driven from the top down. Failing to maintain compliance can and will result in a regulator taking action against an organisation.”
“Device manufacturers should strive for certification to ISO 13485, which covers general regulatory requirements. Device manufacturers must always be vigilant in maintaining compliance with FDA (US Food and Drug Administration), MDD (Medical Device Directive), MDR (Medical Device Regulation) and other regulations, depending on the markets being served.
Furthermore, rigorous clinical evaluations and post-market surveillance are critical to demonstrate the safety and efficacy of medical devices. Companies should collect and analyse real-world data, monitor adverse events, and proactively address any safety concerns.
Finding compliance with safe partners
For advanced medical devices, manufacturers often need to work with external partners to find expertise specific to certain components.
Alleima is an international medical wire forming supplier that works with manufacturers from the design to the production stage. With extensive expertise and a global footprint, Alleima not only works with OEMs to produce medical wire-based components that is perfectly calibrated to the application but also helps partners to navigate regulatory challenges.
The company’s wire-forming facilities in Italy, Germany, Switzerland and USA adhere to ISO 13485 and ISO 9001 (for quality management systems), as well as ISO 14001 and ISO 45001 for environmental management and occupational health/safety respectively. Alleima ESG goals and compliance, including the company’s commitment to the Science Based Target’s initiative, are published on its website www.alleima.com.



