Fortis Life Sciences is a contract development and manufacturing organisation (CDMO) that offers reagents, tools, materials, and custom services for diagnostic manufacturers. Our subsidiary nanoComposix supplies end-to-end lateral flow assay development and manufacturing services, custom conjugation services for lateral flow, and precisely engineered and highly characterised nanoparticles for lateral flow and other diagnostic applications.
nanoComposix is certified according to ISO 13485 (2016) standards and is registered with the US Food and Drug Administration (FDA). Our nanomaterial product portfolio contains a variety of metal nanoparticles many of which can be developed in line with good manufacturing practice (GMP) standards to meet customers’ needs.
Our products and CDMO services are supported by technical teams with extensive expertise in the fabrication and scale-up of nanomaterials. The teams have strong backgrounds in nanotechnology, biology, and chemistry, making them uniquely suited to the challenges of diagnostic applications.
Lateral flow assay development and manufacturing services
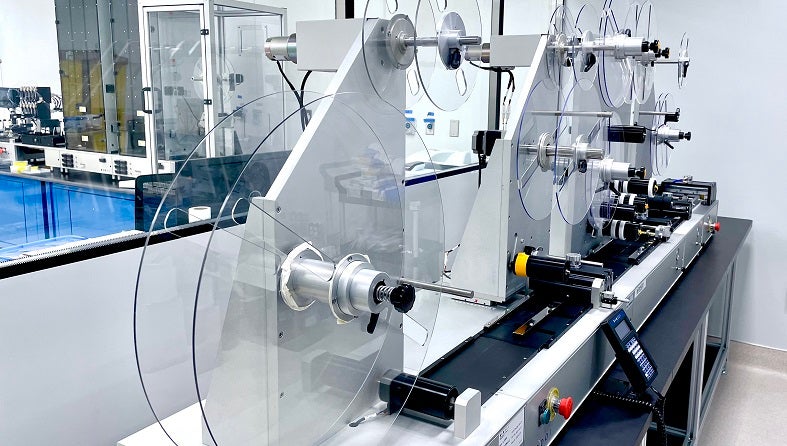
Over the past ten years, nanoComposix has become a leading CDMO service provider helping customers launch products for diagnostic applications in infectious diseases, oncology, veterinary medicine, and food safety testing.
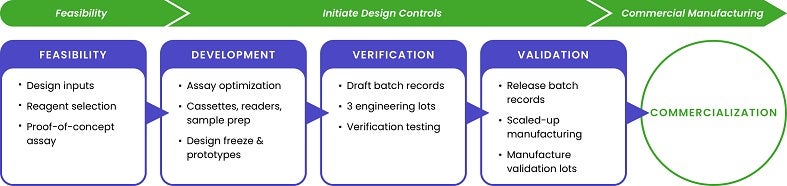
Our services cover all phases of the lateral flow assay development cycle, from feasibility to manufacturing. We leverage our expertise in nanomaterials and comprehensive knowledge of medical devices to develop innovative manufacturing solutions that are tailored to meet our clients’ unique needs.
nanoComposix’s in-house biochemistry experts and commercial-scale manufacturing teams provide unparalleled flexibility from start to finish, helping companies bring new medical device products to market with ease.
Custom conjugation services for lateral flow assays
As a contract developer and manufacturer of lateral flow assays, nanoComposix’s team of experts can help with optimising, scaling up, and producing conjugates at multi-litre scales. Using our ISO 13485:2016 certified quality management system (QMS), we can help develop customised conjugation procedures to meet your requirements for proteins, antibodies, and small molecules.
To enable rapid transfer to manufacturing, nanoComposix’s development activities are vertically integrated into our current GMP (cGMP) facilities. In addition, we can analyse your current conjugation procedures and provide customised solutions to solve any issues.
Design and development of nanoparticles for diagnostics
nanoComposix’s multi-disciplinary team of scientists have backgrounds in chemistry, physics, engineering, and biology. They have worked with more than 100 clients to develop nanomaterials, composites, and formulations with tailored optical, physical, and biofunctional properties.
Our expertise in developing and scaling-up nanomaterials for use in ISO 13485:2016 and cGMP-compliant products makes us uniquely suited to the challenges of diagnostic applications.
nanoComposix’s full range of services includes feasibility synthesis and characterisation; custom particle development and optimisation; scale-up, process development and validation; and cGMP manufacturing.
GMP nanomaterial synthesis and manufacturing
nanoComposix specialises in using nanomaterial components to develop and manufacture devices and drugs. Our team offers support for all stages of the nanoparticle manufacturing process, including proof-of-concept research and development (R&D), scale-up, and cGMP manufacturing.
For products that must be developed in cGMP-compliant quality systems, nanoComposix can also provide support in fabricating and scaling up nanomaterials.
About nanoComposix
nanoComposix was established in 2004 to provide accurate custom nanomaterials for clients worldwide. As an ISO 13485:2016-certified, FDA-registered site, we aim to ensure our products and services are of the highest quality and meet all our customer’s expectations.
We operate from our head office in San Diego, California.