UV Adhesives for In-Vitro Diagnostic Test Assembly

In-vitro diagnostic tests are known to use substrates such as COC / COP thermoplastics or glass that are extremely difficult to bond. Dymax medical device adhesives allow for easier bonding and assembly by using light to quickly cure the adhesive once the adhesive is applied, the manufacturer has unlimited time to adjust the parts. Then, the adhesive can be cured in seconds when exposed to UV / Visible light. These products may also be of interest to manufacturers involved with the design of Covid-19 rapid diagnostic applications.
Additionally, some formulations utilise Dymax patented See-Cure or Ultra-Red® fluorescing technology. Products formulated with Encompass® technology which combines See-Cure and Ultra-red fluorescing technologies into one product for easy visual verification of material placement, cure, and quality inspection, are also available,
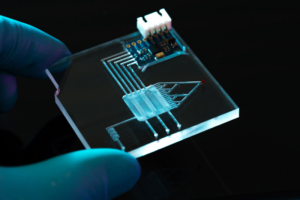
Assembly processes include the manufacturing of diagnostic slides, microplates, silicon wafers, or lab-on-a-chip device, which are used in many medical applications such as blood or tissue testing.
MD® 1172-M-UR
Dymax MD® 1172-M-UR medical device adhesive is designed for rapid assembly of COC, COP, and a variety of other difficult-to-bond plastics typically used in the manufacture of medical devices. Applications include microfluidics, lab-on-a-chip, reservoir and transducer assembly, as well as medical potting. 1172-M-UR features Ultra-Red® technology which enables the adhesive to fluoresce bright red under low-intensity black light (365 nm). This bright red fluorescence contrasts extremely well on plastics that naturally fluoresce blue in colour (like PVC), and greatly assists with a visual inspection of the bond-line area. The product is flexible and ideally suited for bonding a wide variety of substrates including COC, COP, ABS, PC, PMMA, PVC, EVA, PA, PCTG, PEBA, PEI, PET, PETG, PS, PSU, SAN, TPU, CER, AL, and stainless steel.
MD® 1172-M-UR medical device adhesive is in full compliance with the RoHS2 Directives 2015 / 863 / EU.