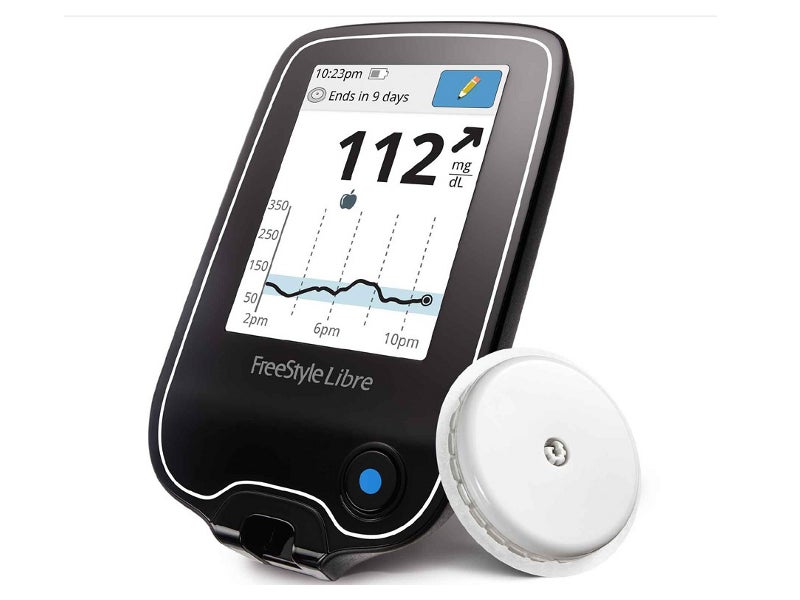
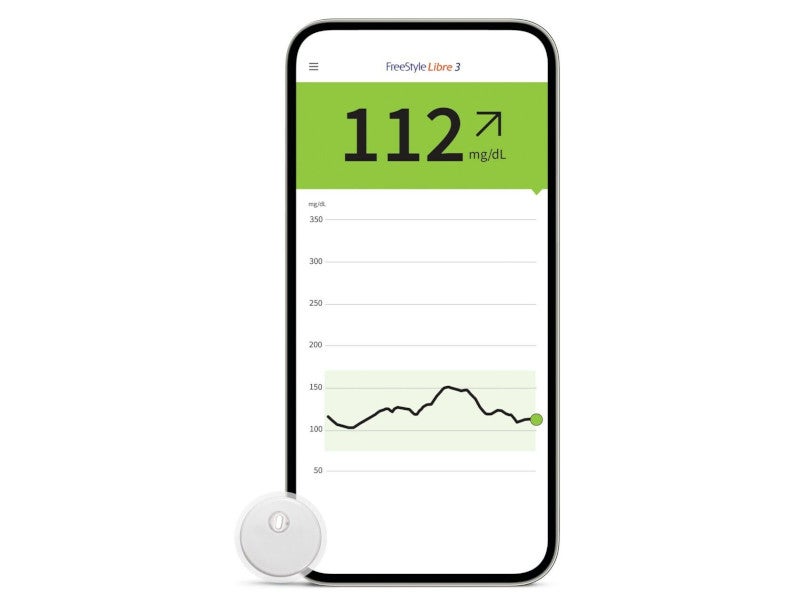
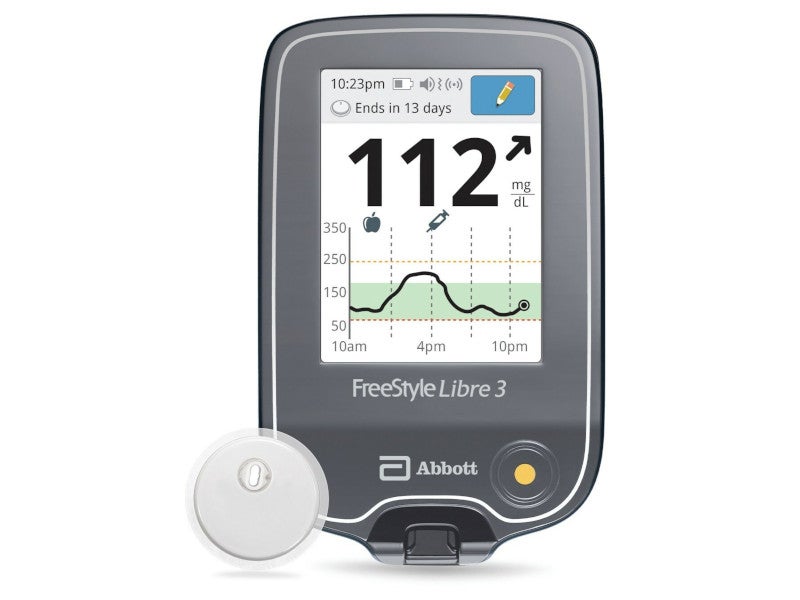
Freestyle Libre® is a continuous blood glucose level monitoring device indicated for the management of diabetes. It consists of a disposable sensor, a patch delivery unit and a reader.
It was developed by Abbott, a medical devices and healthcare company based in the US.
The Freestyle Libre product portfolio includes the FreeStyle Libre 2 and FreeStyle Libre 3 glucose monitoring systems. The FreeStyle Libre 3 system features the world’s smallest and thinnest sensor.
Regulatory approvals
The Freestyle Libre was first launched in Europe in 2014. It received the European CE mark to manage diabetes in children aged between four and 17 years in February 2016.
The US Food and Drug Administration (FDA) approved the device to monitor trends and patterns of blood glucose levels in diabetes patients in September 2017.
Abbott filed a supplementary pre-market approval application to the FDA in April 2018 to expand the time that the device’s sensor should be worn to 14 days, with sensor warm-up time reduced to one hour. The device was approved for the expanded indication in June 2018.
FreeStyle Libre 2, the next generation of the FreeStyle Libre glucose monitoring device, was approved by the FDA in June 2020 for adults and children aged four years and above with diabetes.
Abbott received approval for the newest version of the FreeStyle Libre glucose monitoring device, FreeStyle Libre 3, in September 2020 in Europe and in May 2022 in the US.
The FreeStyle Libre 3 reader, a handheld device that displays real-time glucose readings, was approved by the FDA in April 2023.
In March 2023, the device received FDA approval for integration with automated insulin delivery systems.
Freestyle Libre system component details
Freestyle Libre comprises an electrochemical glucose sensor tail and an electronics system. The tail is implanted into the subcutaneous tissue of the back of the upper arm, while the electronics patch is stuck to the back of the arm using medical-grade adhesive.
The device is inserted using the patch delivery unit. Putting the sensor tail in place approximately 5.5mm deep into the skin, it encompasses an applicator and a sensor pack, which is sterilised by e-beam.
The electronics system provides power to the unit and transmits data to a hand-held reader, which connects to the sensor through radiofrequency waves. One reader can collect blood glucose data from multiple sensors.
Functioning and other features of Freestyle Libre system
The thin flexible metal fibre of Freestyle Libre’s sensor generates electrical signals that correspond to the patient’s glucose levels. When the patient scans the reader over the sensor, it records these electrical signals to provide a blood glucose reading.
A FreeStyle LibreLink app can be downloaded to the user’s smartphone, which allows their phone to act as the reader. The app can be connected to the LibreLinkUp app for remote monitoring and sharing.
The device can be connected to different digital solutions such as LibreView to make glucose monitoring easy. LibreView is a free, cloud-based system for diabetes management. It provides reports to identify glucose level patterns.
Benefits of the Freestyle Libre system
The Freestyle Libre continuous glucose monitoring system helps diabetes patients to instantly check their blood glucose level at any time of the day without the traditional finger prick testing system.
The sensor is factory calibrated and does not require user calibration. It should be worn continuously for 14 days to ensure accurate readings are taken.
The sensor is water-resistant and can be used when bathing or swimming. The reader can store up to 90 days’ worth of data, which is useful for checking long-term glucose trends to identify concerns or improvements.
The system also provides accurate blood glucose reading for pregnant women without the need for a finger prick test, reducing the risk of adverse events in patients.